A new antibiotic, A, which is an acid, can readily be oxidized by hot aqueous permanganate; the latter is reducedto manganous ion, Mn2+. The following experiments havebeen performed with A: (a) 0.293 g A consumes just18.3 mL of 0.080 M KMnO4; (b) 0.385 g A is just neutralized by 15.7 mL of 0.490 M NaOH. What can you conclude about the molecular weight of A from (a), from (b), and from both considered together?
A new antibiotic, A, which is an acid, can readily be oxidized by hot aqueous permanganate; the latter is reduced
to manganous ion, Mn2+. The following experiments have
been performed with A: (a) 0.293 g A consumes just
18.3 mL of 0.080 M KMnO4; (b) 0.385 g A is just neutralized by 15.7 mL of 0.490 M NaOH. What can you conclude about the molecular weight of A from (a), from (b), and from both considered together?
In a titration experiment, at neutralization point, no of moles multiplied by n-factor of titrant and analyte becomes equal.
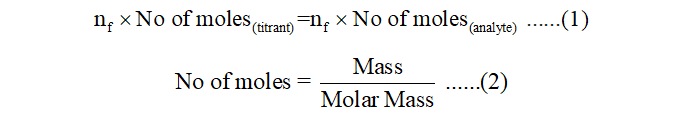
(a)
When acid is titrated with KMnO4, KMnO4 reduces to Mn2+ and acid gets oxidized. Since the oxidation state of KMnO4 changes from +7 to +2, thus n-factor of KMnO4 will be change in oxidation state i.e. +7-(+2) = 4. Thus, from equation (1), we get
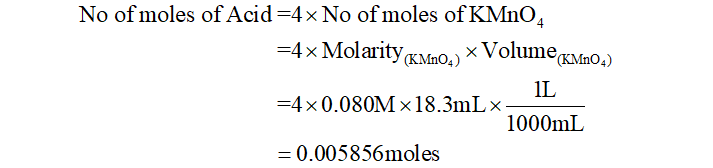
Putting the value of no of moles in equation (2), we can find the molar mass of acid as:
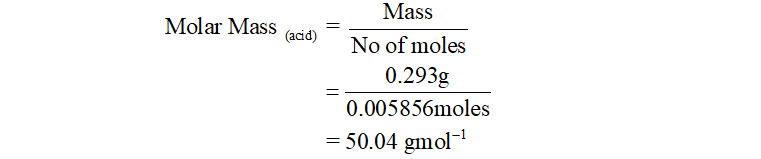
Step by step
Solved in 4 steps with 5 images









