A person consumes 100 micrograms of a tracer chemical. Assume that the person is able to collect all of the tracer in their urine (and therefore measure the amount that has come out of the body), as well as the concentration in the blood, as a function of time (see table below). a Is a first-order rate constant appropriate for describing the process of elimination via the kidneys? Justify your answer. b Assuming that the answer to a is ”yes,” find the rate constant k and the total blood volume V from these data. c is the value of V that you determined equal to the blood volume of the person? Does this make sense to you? If not, what does V represent?
A person consumes 100 micrograms of a tracer chemical. Assume that the person is able to collect all of the tracer in their urine (and therefore measure the amount that has come out of the body), as well as the concentration in the blood, as a function of time (see table below). a Is a first-order rate constant appropriate for describing the process of elimination via the kidneys? Justify your answer. b Assuming that the answer to a is ”yes,” find the rate constant k and the total blood volume V from these data. c is the value of V that you determined equal to the blood volume of the person? Does this make sense to you? If not, what does V represent?
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter25: Body Fluids
Section: Chapter Questions
Problem 25.33E
Related questions
Question
A person consumes 100 micrograms of a tracer chemical. Assume that the person is able to collect all
of the tracer in their urine (and therefore measure the amount that has come out of the body), as well
as the concentration in the blood, as a function of time (see table below).
a Is a first-order rate constant appropriate for describing the process of elimination via the kidneys?
Justify your answer.
b Assuming that the answer to a is ”yes,” find the rate constant k and the total blood volume V from
these data.
c is the value of V that you determined equal to the blood volume of the person? Does this make
sense to you? If not, what does V represent?
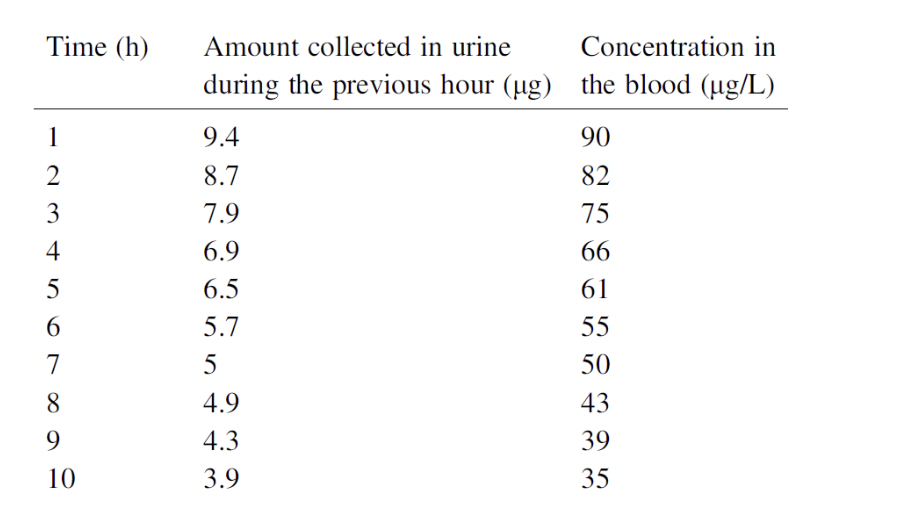
Transcribed Image Text:Time (h)
Amount collected in urine
Concentration in
during the previous hour (ug) the blood (ug/L)
1
9.4
90
8.7
82
3
7.9
75
4
6.9
66
6.5
61
5.7
55
7
5
50
8.
4.9
43
9
4.3
39
10
3.9
35
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole