A person makes a quantity of iced tea by mixing 290 g of hot tea (essentially water) with an equal mass of ice at its melting point. Assume the mixture has negligible energy exchanges with its environment. (a) If the tea's initial temperature is T- 87°C, when thermal equilibrium is reached what are the mixture's temperature T; and (b) the remaining mass m;of ice? If T, - 51°C, when thermal t of equilibrium is reached what are (c) T; and (d) m2 The specific heat of water is 4186 J/kg-K. The latent heat of fusion is 333 kJ/kg. (a) Number i Units (b) Number Units (c) Number Units (d) Number Units
A person makes a quantity of iced tea by mixing 290 g of hot tea (essentially water) with an equal mass of ice at its melting point. Assume the mixture has negligible energy exchanges with its environment. (a) If the tea's initial temperature is T- 87°C, when thermal equilibrium is reached what are the mixture's temperature T; and (b) the remaining mass m;of ice? If T, - 51°C, when thermal t of equilibrium is reached what are (c) T; and (d) m2 The specific heat of water is 4186 J/kg-K. The latent heat of fusion is 333 kJ/kg. (a) Number i Units (b) Number Units (c) Number Units (d) Number Units
Physics for Scientists and Engineers: Foundations and Connections
1st Edition
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Katz, Debora M.
Chapter21: Heat And The First Law Of Thermodynamics
Section: Chapter Questions
Problem 31PQ: Consider the latent heat of fusion and the latent heat of vaporization for H2O, 3.33 105 J/kg and...
Related questions
Question
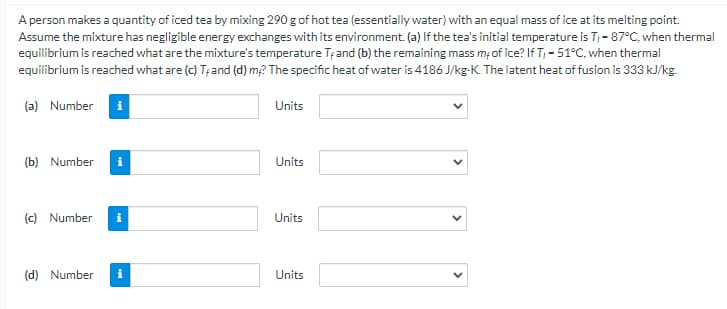
Transcribed Image Text:A person makes a quantity of iced tea by mixing 290 g of hot tea (essentially water) with an equal mass of ice at its melting point.
Assume the mixture has negligible energy exchanges with its environment. (a) If the tea's initial temperature is T,- 87°C, when thermal
eqilibrium is reached what are the mixture's temperature Tțand (b) the remaining mass mof ice? If T, - 51°C, when thermal
equilibrium is reached what are (c) T;and (d) m2 The specific heat of water is 4186 J/kg-K. The latent heat of fusion is 333 kJ/kg.
(a) Number
Units
(b) Number
Units
(c) Number
Units
(d) Number
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning