a) Potentiometric techniques require the usage of different reference electrodes. One of such electrodes is the saturated calomel reference electrode (SCE). i. Draw a labelled diagram showing the saturated calomel reference electrode (SCE). Why is it referred to as a saturated calomel reference electrode? ii. iii. Write the ha f-cell reaction for the SCE iv. Find the cell potential for an electrochemical cell given that the standard reduction 0.25V Ni/Ni (s) potential for nickel is and the standard reduction potential for SCE is Escr +0.244V
a) Potentiometric techniques require the usage of different reference electrodes. One of such electrodes is the saturated calomel reference electrode (SCE). i. Draw a labelled diagram showing the saturated calomel reference electrode (SCE). Why is it referred to as a saturated calomel reference electrode? ii. iii. Write the ha f-cell reaction for the SCE iv. Find the cell potential for an electrochemical cell given that the standard reduction 0.25V Ni/Ni (s) potential for nickel is and the standard reduction potential for SCE is Escr +0.244V
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.23QAP
Related questions
Question
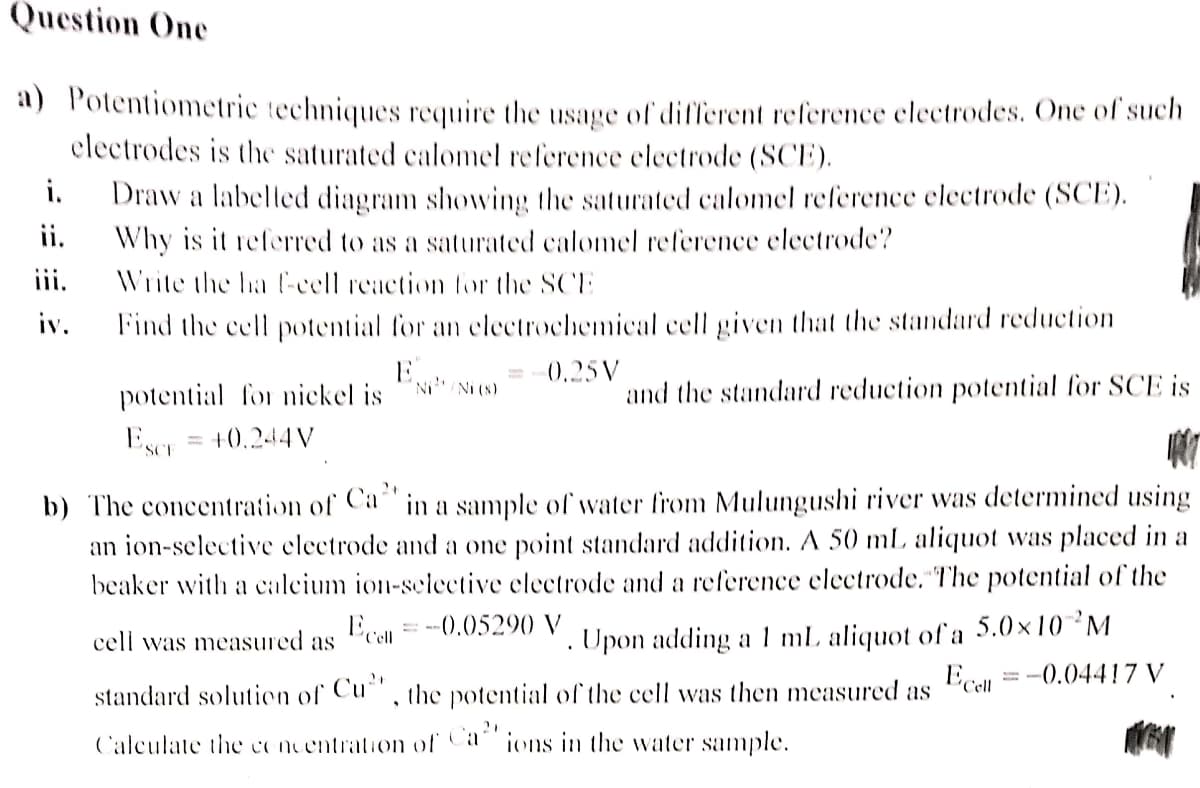
Transcribed Image Text:Question One
a) Potentiometric techniques require the usage of different reference electrodes. One of such
electrodes is the saturated calomel reference electrode (SCE).
i.
Draw a labelled diagram showing the saturated calomel reference electrode (SCE).
Why is it referred to as a saturated calomel reference electrode?
ii.
iii.
Write the ha f-cell reaction for the SCE
iv.
Find the cell potential for an electrochemical cell given that the standard reduction
E...
0.25V
'Ni/Ni (s)
and the standard reduction potential for SCE is
potential for nickel is
Wer
Escr
+0.244V
2+
b) The concentration of Ca in a sample of water from Mulungushi river was determined using
an ion-selective electrode and a one point standard addition. A 50 mL aliquot was placed in a
beaker with a calcium ion-selective electrode and a reference electrode. The potential of the
Ecell
= -0.05290 V
.
cell was measured as
Ecell
= -0.04417 V
Upon adding a 1 mL aliquot of a 5.0×10 ²M
standard solution of Cu", the potential of the cell was then measured as
Calculate the concentration of Ca ions in the water sample.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning