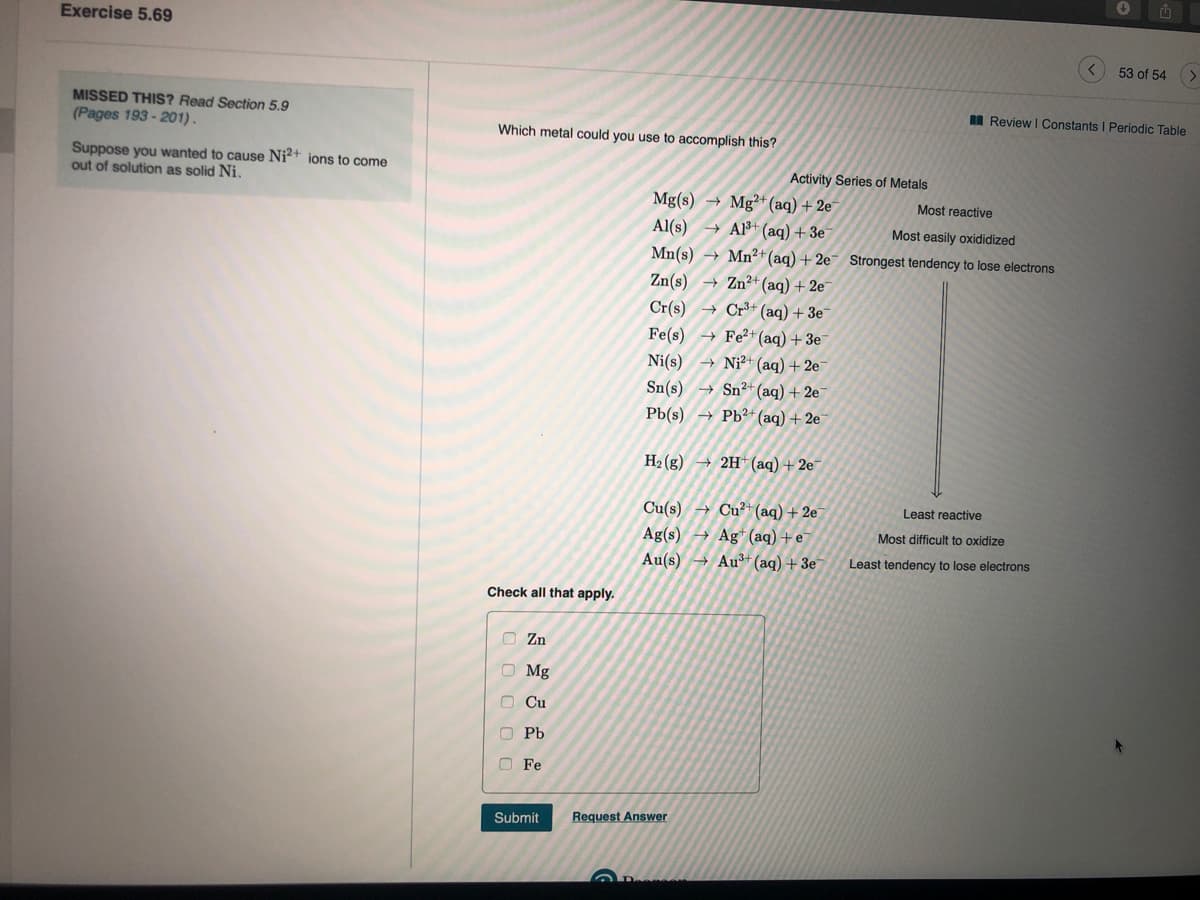
A Review I Consta MISSED THIS? Read Section 5.9 (Pages 193 - 201). Which metal could you use to accomplish this? Suppose you wanted to cause Ni²+ ions to come out of solution as solid Ni. Activity Series of Metals Mg(s) → Mg²+ (aq) + 2e Al(s) → Al+ (aq) + 3e Most reactive Most easily oxididized Mn(s) → Mn²+(aq) + 2e¯ Strongest tendency to lose electrons → Zn²+(aq) + 2e Cr(s) + Cr³+ (aq) + 3e¯ Fe2+ (aq) + 3e Zn(s) Fe(s) Ni?+ (aq) + 2e - Sn2+ (aq) + 2e Pb(s) → Pb²+ (aq) + 2e¯ Ni(s) Sn(s) H2 (g) → 2H* (aq) + 2e 4 Cu²+(aq) + 2e Ag (aq) +e Au(s) → Au+ (aq) + 3e Cu(s) Least reactive Ag(s) Most difficult to oxio Least tendency to lose electrons Check all that apply. O Zn O Mg O Cu O Pb O Fe Submit Request Answer
A Review I Consta MISSED THIS? Read Section 5.9 (Pages 193 - 201). Which metal could you use to accomplish this? Suppose you wanted to cause Ni²+ ions to come out of solution as solid Ni. Activity Series of Metals Mg(s) → Mg²+ (aq) + 2e Al(s) → Al+ (aq) + 3e Most reactive Most easily oxididized Mn(s) → Mn²+(aq) + 2e¯ Strongest tendency to lose electrons → Zn²+(aq) + 2e Cr(s) + Cr³+ (aq) + 3e¯ Fe2+ (aq) + 3e Zn(s) Fe(s) Ni?+ (aq) + 2e - Sn2+ (aq) + 2e Pb(s) → Pb²+ (aq) + 2e¯ Ni(s) Sn(s) H2 (g) → 2H* (aq) + 2e 4 Cu²+(aq) + 2e Ag (aq) +e Au(s) → Au+ (aq) + 3e Cu(s) Least reactive Ag(s) Most difficult to oxio Least tendency to lose electrons Check all that apply. O Zn O Mg O Cu O Pb O Fe Submit Request Answer
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter19: Oxidation-reduction(electron Transfer) Reactions
Section: Chapter Questions
Problem 8PE
Related questions
Question
100%
Transcribed Image Text:Exercise 5.69
53 of 54
MISSED THIS? Read Section 5.9
I Review I Constants I Periodic Table
(Pages 193 - 201).
Which metal could you use to accomplish this?
Suppose you wanted to cause Ni?+ ions to come
out of solution as solid Ni.
Activity Series of Metals
Mg(s) → Mg²+*(aq) + 2e
Al(s) → Al³+ (aq) + 3e
Mn(s) → Mn²+(aq) + 2e¯ Strongest tendency to lose electrons
Most reactive
Most easily oxididized
→ Zn²+(aq) + 2e
Cr+ (aq) + 3e
+ Fe²+(aq) +3e
Zn(s)
Cr(s)
Fe(s)
Ni²+ (aq) + 2e¯
→ Sn²*(aq) + 2e
Pb(s) → Pb²+(aq) + 2e¯
Ni(s)
Sn(s)
H2 (g
→ 2H (aq)+ 2e
Cu(s)
Ag(s)
Au(s) → Au+ (aq) + 3e
Cu²+(aq) + 2e
→ Ag* (aq)+e
Least reactive
Most difficult to oxidize
Least tendency to lose electrons
Check all that apply.
O Zn
O Mg
O Cu
O Pb
O Fe
Submit
Request Answer
Expert Solution
Step 1 Analysis

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning