Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 4E
Related questions
Question
Transcribed Image Text:* 00
R.
P.
6
8.
7.
9.
5.
4.
24
&
MacBook Pro
O N30
O NO2
O'N O
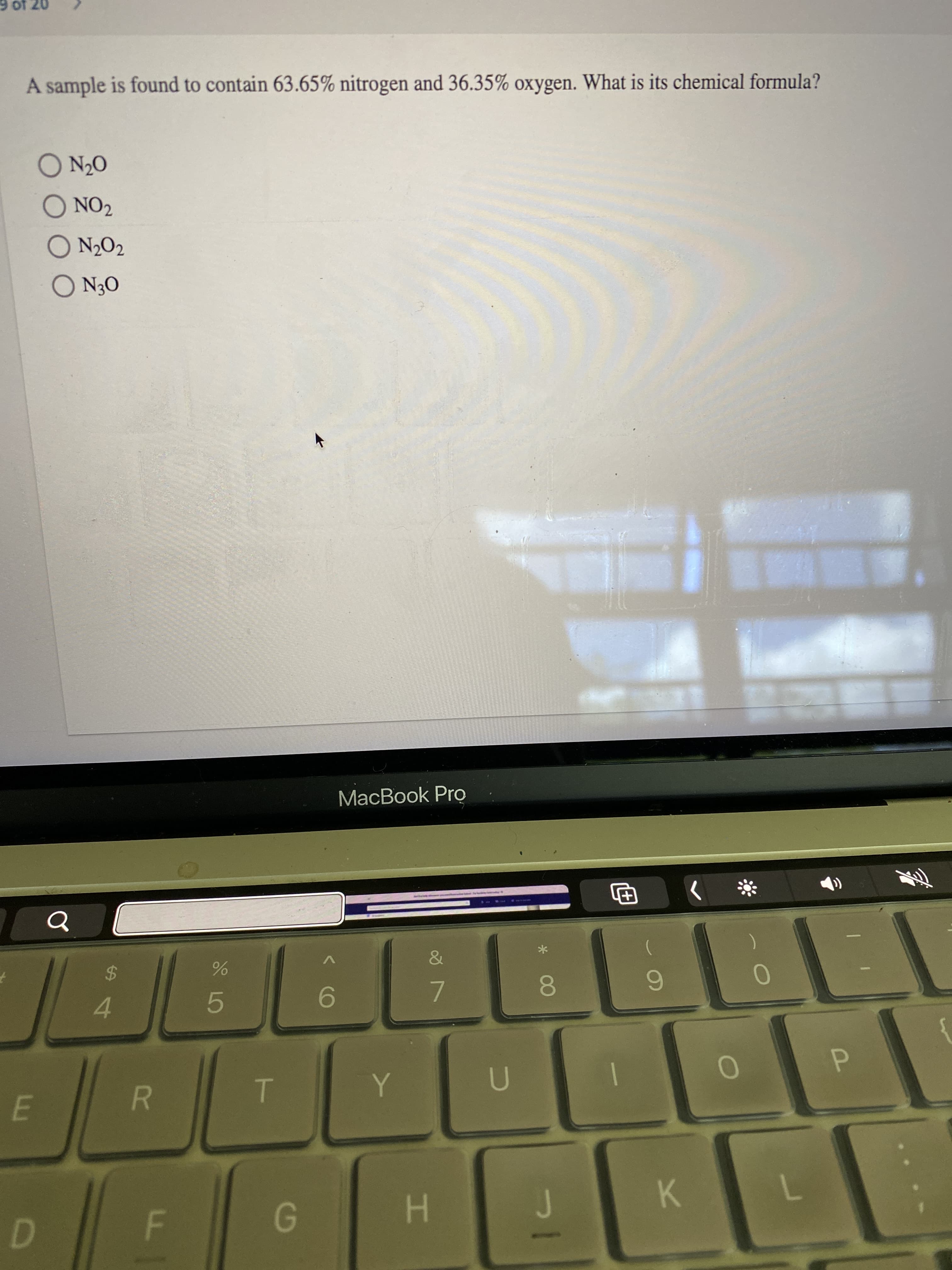
A sample is found to contain 63.65% nitrogen and 36.35% oxygen. What is its chemical formula?
OZ 10 6
Expert Solution
Step 1
Emperical formula can be determined from the % of different elements present in a molecule. If vapour density/molecular mass of compound is known then we can also find out the molecular formula.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning