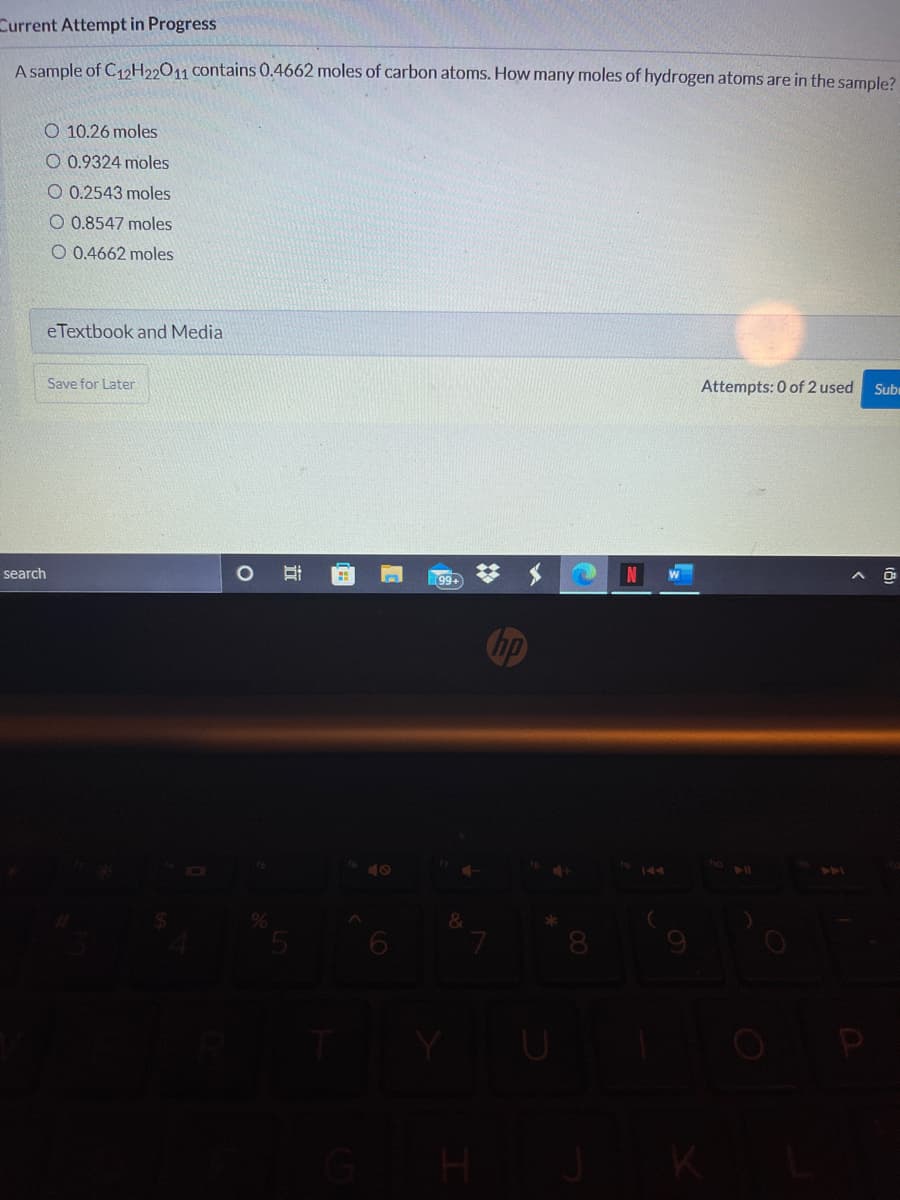
A sample of C12H22011 contains 0.4662 moles of carbon atoms. How many moles of hydrogen atoms are in the sample? O 10.26 moles O 0.9324 moles O 0.2543 moles O 0.8547 moles O 0.4662 moles
A sample of C12H22011 contains 0.4662 moles of carbon atoms. How many moles of hydrogen atoms are in the sample? O 10.26 moles O 0.9324 moles O 0.2543 moles O 0.8547 moles O 0.4662 moles
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 3ALQ: True or false? The atom with the largest subscript in a formula is die atom with the largest percent...
Related questions
Question
Transcribed Image Text:Current Attempt in Progress
A sample of C12H22011 contains 0.4662 moles of carbon atoms. How many moles of hydrogen atoms are in the sample?
O 10.26 moles
O 0.9324 moles
O 0.2543 moles
O 0.8547 moles
O 0.4662 moles
eTextbook and Media
Save for Later
Attempts: 0 of 2 used
Sub
search
99+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.