49. How many moles are there in 500 g indigo, C16H10N202, a blue dye. c. 1.15 X10E24 mol a. 1.90 mol b. 131000 mol d. 7.89 ×10E28 mol 50. Calculate the number of molecules of a 2.00 g Valium, C15H13CIN20. a. 4.43 ×10E21 b. 8.19 ×10E25 C. 3.28 ×10E26 d. 9.03 ×10E29
49. How many moles are there in 500 g indigo, C16H10N202, a blue dye. c. 1.15 X10E24 mol a. 1.90 mol b. 131000 mol d. 7.89 ×10E28 mol 50. Calculate the number of molecules of a 2.00 g Valium, C15H13CIN20. a. 4.43 ×10E21 b. 8.19 ×10E25 C. 3.28 ×10E26 d. 9.03 ×10E29
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter5: Stoichiometry
Section: Chapter Questions
Problem 16ALQ: Consider the equation 2A + B . A2B. If you mix 1.0 mole of A with 1.0 mole of B, what amount (moles)...
Related questions
Question
please answer all :(
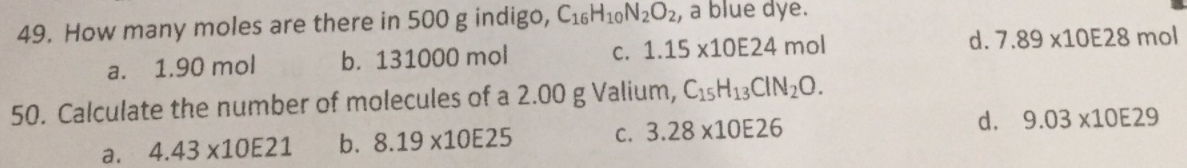
Transcribed Image Text:49. How many moles are there in 500 g indigo, C16H10N2O2, a blue dye.
a.
1.90 mol
b. 131000 mol
c. 1.15 X10E24 mol
d. 7.89 ×10E28 mol
50. Calculate the number of molecules of a 2.00 g Valium, C15H13CIN20.
a. 4.43 ×1OE21
b. 8.19 ×10E25
c. 3.28 ×10E26
d. 9.03 X10E29
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning