A sample of iron was heated to 112.0 °C, then placed into 260 g of water at 22.8 °C. The temperature of the water rose to 29.5 °C. How many grams of iron were in the sample? (Refer to a table of specific heat of selected substances.) TABLE 4.3 | Specific Heat of Selected Substances Substance Specific heat (J/g°C) Specific heat (cal/g°C) Water 4.184 1.000 Ethyl alcohol Ice Aluminum 2.138 2.059 0.511 0.492 0.215 0.900 Iron 0.473 0.113 0.385 0.0921 Copper Gold 0.131 0.0312
A sample of iron was heated to 112.0 °C, then placed into 260 g of water at 22.8 °C. The temperature of the water rose to 29.5 °C. How many grams of iron were in the sample? (Refer to a table of specific heat of selected substances.) TABLE 4.3 | Specific Heat of Selected Substances Substance Specific heat (J/g°C) Specific heat (cal/g°C) Water 4.184 1.000 Ethyl alcohol Ice Aluminum 2.138 2.059 0.511 0.492 0.215 0.900 Iron 0.473 0.113 0.385 0.0921 Copper Gold 0.131 0.0312
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 20E: A 45-g aluminum spoon (specific heat 0.88 J/g C) at 24 C is placed in 180 mL (180 g) of coffee at 85...
Related questions
Question
100%
Hello! I'm lost on how to complete this question.
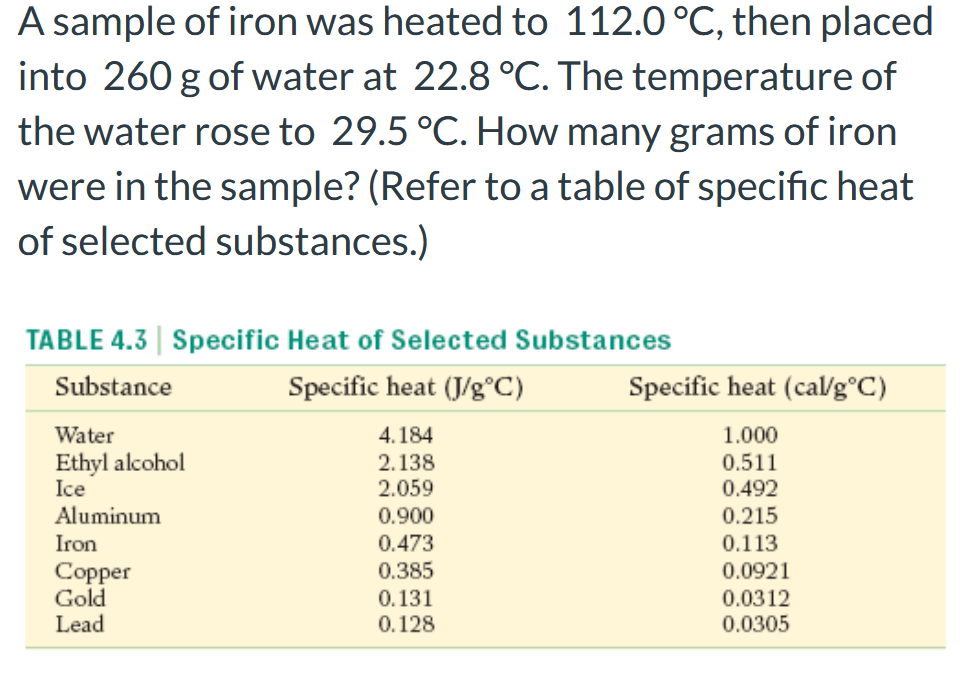
Transcribed Image Text:A sample of iron was heated to 112.0 °C, then placed
into 260 g of water at 22.8 °C. The temperature of
the water rose to 29.5 °C. How many grams of iron
were in the sample? (Refer to a table of specific heat
of selected substances.)
TABLE 4.3 | Specific Heat of Selected Substances
Substance
Specific heat (J/g°C)
Specific heat (cal/g°C)
1.000
0.511
Water
4.184
Ethyl alcohol
Ice
Aluminum
2.138
2.059
0.492
0.900
0.215
Iron
0.113
0.0921
0.473
0.385
Copper
Gold
Lead
0.131
0.128
0.0312
0.0305
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning