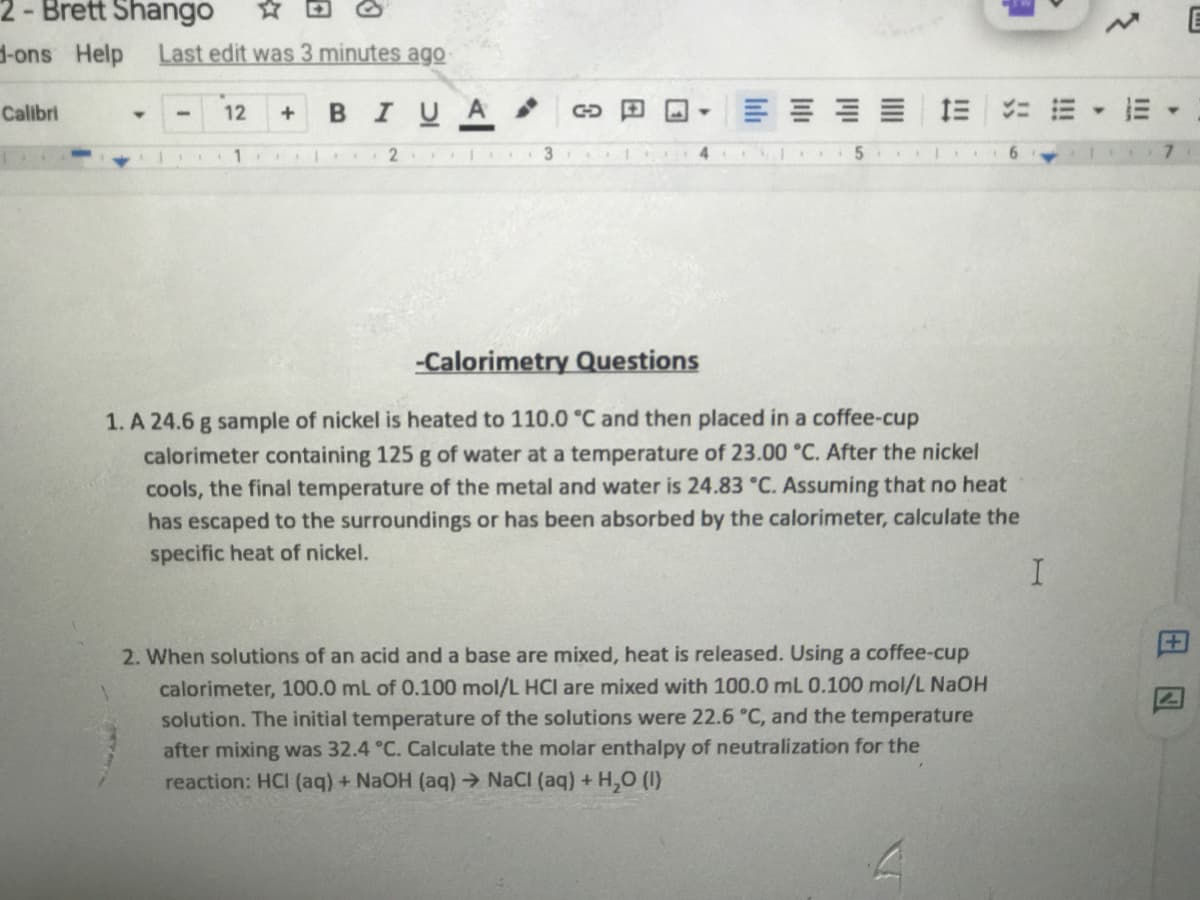
-Calorimetry Questions 1. A 24.6 g sample of nickel is heated to 110.0 °C and then placed in a coffee-cup calorimeter containing 125 g of water at a temperature of 23.00 °C. After the nickel cools, the final temperature of the metal and water is 24.83 °C. Assuming that no heat has escaped to the surroundings or has been absorbed by the calorimeter, calculate the specific heat of nickel. 2. When solutions of an acid and a base are mixed, heat is released. Using a coffee-cup calorimeter, 100.0 mL of 0.100 mol/L HCI are mixed with 100.0 mL 0.100 mol/L NaOH solution. The initial temperature of the solutions were 22.6 °C, and the temperature after mixing was 32.4 °C. Calculate the molar enthalpy of neutralization for the reaction: HCI (aq) + NaOH (aq) → NaCI (aq) + H,0 (1)
-Calorimetry Questions 1. A 24.6 g sample of nickel is heated to 110.0 °C and then placed in a coffee-cup calorimeter containing 125 g of water at a temperature of 23.00 °C. After the nickel cools, the final temperature of the metal and water is 24.83 °C. Assuming that no heat has escaped to the surroundings or has been absorbed by the calorimeter, calculate the specific heat of nickel. 2. When solutions of an acid and a base are mixed, heat is released. Using a coffee-cup calorimeter, 100.0 mL of 0.100 mol/L HCI are mixed with 100.0 mL 0.100 mol/L NaOH solution. The initial temperature of the solutions were 22.6 °C, and the temperature after mixing was 32.4 °C. Calculate the molar enthalpy of neutralization for the reaction: HCI (aq) + NaOH (aq) → NaCI (aq) + H,0 (1)
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 109AE: A sample of nickel is heated to 99.8C and placed in a coffee-cup calorimeter containing 150.0 g...
Related questions
Question
Transcribed Image Text:- Brett Shango
☆回
d-ons Help
Last edit was 3 minutes ago
BIUA
三=山 三
Calibri
12
-Calorimetry Questions
1. A 24.6 g sample of nickel is heated to 110.0 °C and then placed in a coffee-cup
calorimeter containing 125 g of water at a temperature of 23.00 °C. After the nickel
cools, the final temperature of the metal and water is 24.83 °C. Assuming that no heat
has escaped to the surroundings or has been absorbed by the calorimeter, calculate the
specific heat of nickel.
2. When solutions of an acid and a base are mixed, heat is released. Using a coffee-cup
calorimeter, 100.0 mL of 0.100 mol/L HCI are mixed with 100.0 mL 0.100 mol/L NaOH
solution. The initial temperature of the solutions were 22.6 °C, and the temperature
after mixing was 32.4 °C. Calculate the molar enthalpy of neutralization for the
reaction: HCI (aq) + NaOH (aq)→ NaCI (aq) + H,O (I)
田回
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning