A sample of lead is heated and placed in a 151.0 g container of water at 22.0 oC. The final temperature of the lead and water is 44.0 oC. Assuming no heat loss, what amount of heat released from the lead sample?
A sample of lead is heated and placed in a 151.0 g container of water at 22.0 oC. The final temperature of the lead and water is 44.0 oC. Assuming no heat loss, what amount of heat released from the lead sample?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter11: Liquids And Solids
Section: Chapter Questions
Problem 11.98QE
Related questions
Question
A sample of lead is heated and placed in a 151.0 g container of water at 22.0 oC. The final temperature of the lead and water is 44.0 oC. Assuming no heat loss, what amount of heat released from the lead sample?
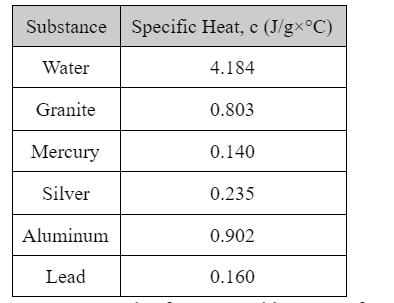
Transcribed Image Text:Substance Specific Heat, c (J/g×°C)
Water
4.184
Granite
0.803
Mercury
0.140
Silver
0.235
Aluminum
0.902
Lead
0.160
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co