A sample of pure aluminum has a mass of 10.1 g. Calculate the HINT (a) moles in the sample moles (b) aluminum atoms in the sample atoms d Holn? Read It Watch It
A sample of pure aluminum has a mass of 10.1 g. Calculate the HINT (a) moles in the sample moles (b) aluminum atoms in the sample atoms d Holn? Read It Watch It
Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 70P: Using a numerical integration method such as Simpson's rule, find the fraction of molecules in a...
Related questions
Question
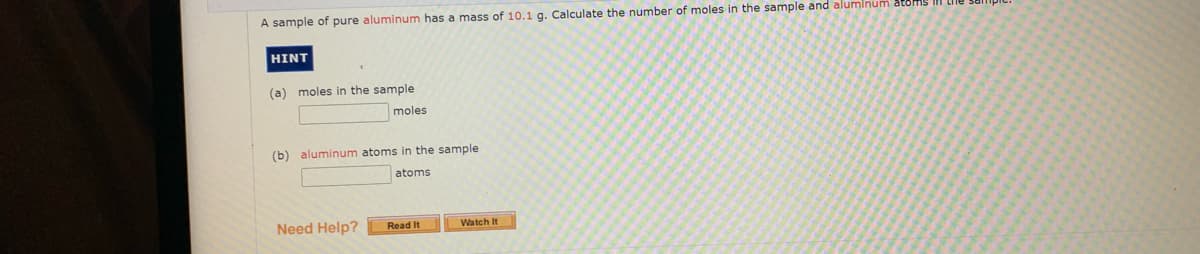
Transcribed Image Text:A sample of pure aluminum has a mass of 10.1 g. Calculate the number of moles in the sample and aluminum
HINT
(a)
moles in the sample
moles
(b) aluminum atoms in the sample
atoms
Need Help?
Read It
Watch It
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning