A soil was measured for cation exchange by leaching 15 g of soil with 80 mL of solution of 1 M ammonium (NH4*) acetate solution buffered at pH 7. The leachate solution was analyzed and found to have 115 mg/L of Ca and 52 mg/L of Mg. The molecular weights are: Ca = 40.1 g/mol; Mg = 24.3 g/mol. 1 mole = 0.01 centimoles. (Watch unit conversions and significant figures!) (a) Calculate the equivalent moles of charge per kg of soil contributed by each cation (Ca and Mg) expressed as centimoles of charge per kilogram of soil (cmol, kgʻ1).
A soil was measured for cation exchange by leaching 15 g of soil with 80 mL of solution of 1 M ammonium (NH4*) acetate solution buffered at pH 7. The leachate solution was analyzed and found to have 115 mg/L of Ca and 52 mg/L of Mg. The molecular weights are: Ca = 40.1 g/mol; Mg = 24.3 g/mol. 1 mole = 0.01 centimoles. (Watch unit conversions and significant figures!) (a) Calculate the equivalent moles of charge per kg of soil contributed by each cation (Ca and Mg) expressed as centimoles of charge per kilogram of soil (cmol, kgʻ1).
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.21QAP
Related questions
Question
D10)
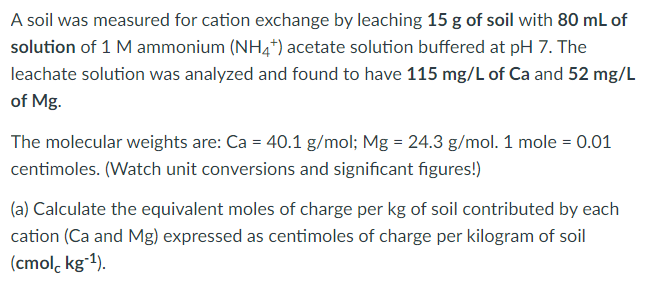
Transcribed Image Text:A soil was measured for cation exchange by leaching 15 g of soil with 80 mL of
solution of 1 M ammonium (NH4*) acetate solution buffered at pH 7. The
leachate solution was analyzed and found to have 115 mg/L of Ca and 52 mg/L
of Mg.
The molecular weights are: Ca = 40.1 g/mol; Mg = 24.3 g/mol. 1 mole = 0.01
centimoles. (Watch unit conversions and significant figures!)
(a) Calculate the equivalent moles of charge per kg of soil contributed by each
cation (Ca and Mg) expressed as centimoles of charge per kilogram of soil
(cmol, kgʻ1).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
