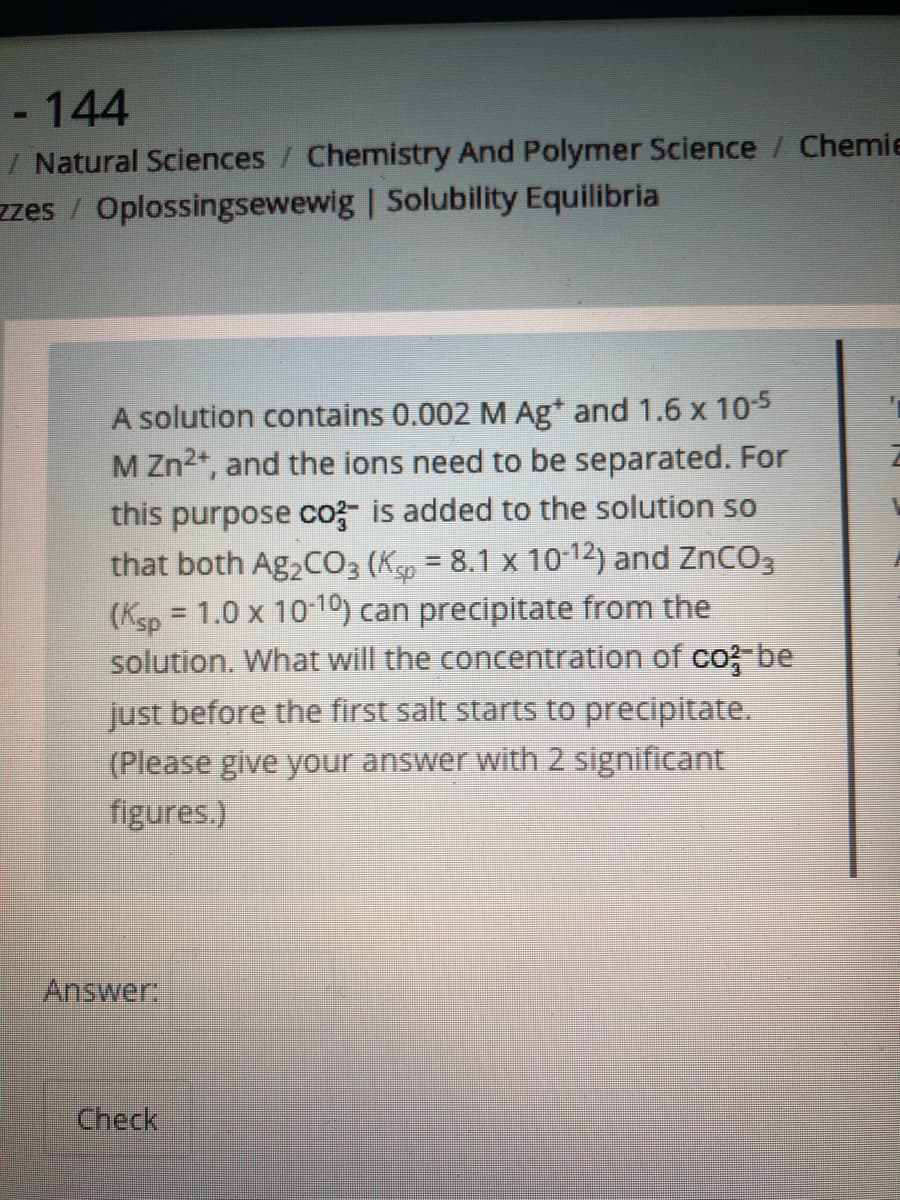
A solution contains 0.002 M Ag* and 1.6 x 105 M Zn2+, and the ions need to be separated. For this purpose co- is added to the solution so that both Ag2CO3 (Ksp = 8.1 x 10-12) and ZnCO3 (Ksp = 1.0 x 10 10) can precipitate from the solution. What will the concentration of co? be just before the first salt starts to precipitate. (Please give your answer with 2 significant figures.) %3D Answer
A solution contains 0.002 M Ag* and 1.6 x 105 M Zn2+, and the ions need to be separated. For this purpose co- is added to the solution so that both Ag2CO3 (Ksp = 8.1 x 10-12) and ZnCO3 (Ksp = 1.0 x 10 10) can precipitate from the solution. What will the concentration of co? be just before the first salt starts to precipitate. (Please give your answer with 2 significant figures.) %3D Answer
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
Transcribed Image Text:- 144
/ Natural Sciences / Chemistry And Polymer Science / Chemie
zzes / Oplossingsewewig | Solubility Equilibria
A solution contains 0.002 M Ag* and 1.6 x 105
M Zn2*, and the ions need to be separated. For
this purpose co- is added to the solution so
that both Ag,CO3 (Ksp = 8.1 x 10-12) and ZnCO3
(Ksp = 1.0 x 10-10) can precipitate from the
solution. What will the concentration of co; be
just before the first salt starts to precipitate.
(Please give your answer with 2 significant
figures.)
Answer
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

