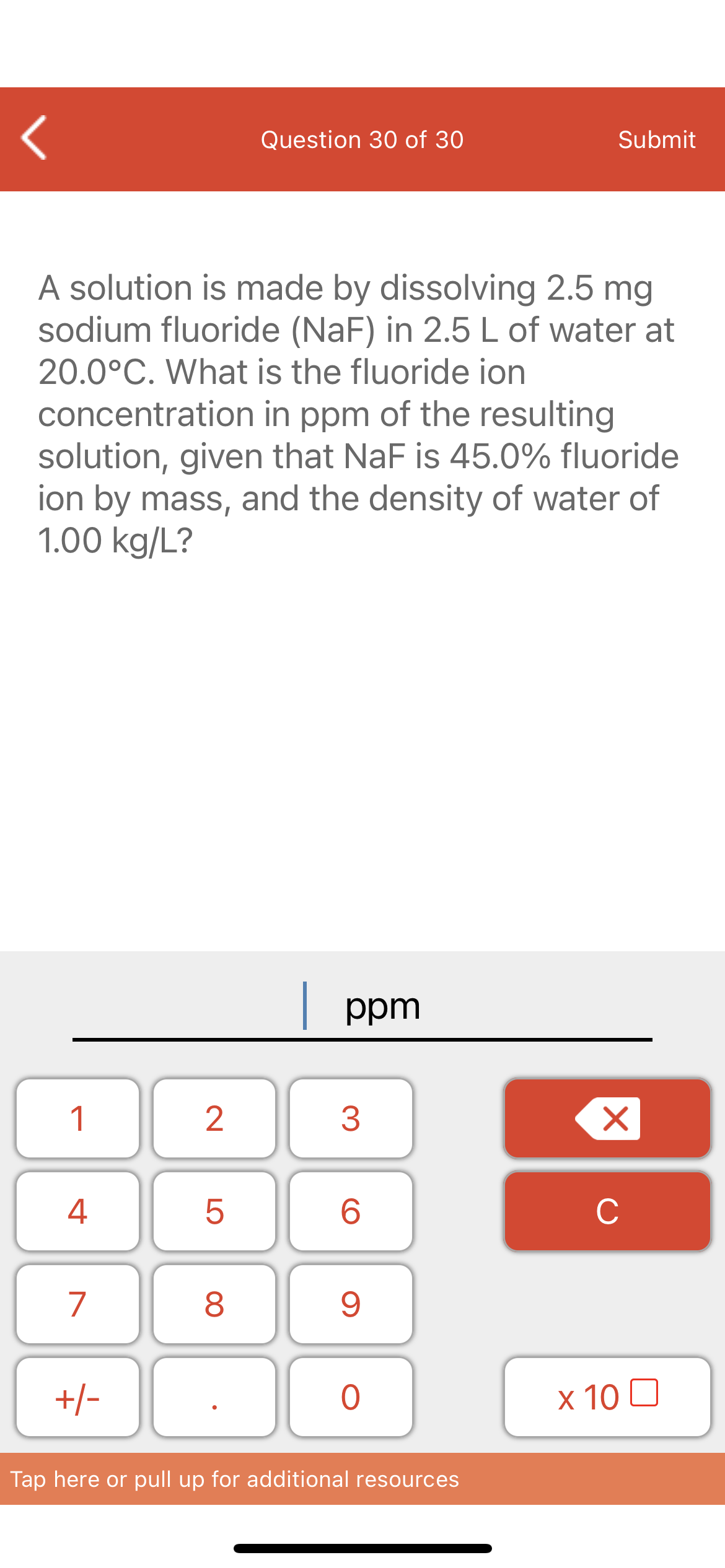
A solution is made by dissolving 2.5 mg sodium fluoride (NaF) in 2.5 L of water at 20.0°C. What is the fluoride ion concentration in ppm of the resulting solution, given that NaF is 45.0% fluoride ion by mass, and the density of water of 1.00 kg/L?
A solution is made by dissolving 2.5 mg sodium fluoride (NaF) in 2.5 L of water at 20.0°C. What is the fluoride ion concentration in ppm of the resulting solution, given that NaF is 45.0% fluoride ion by mass, and the density of water of 1.00 kg/L?
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter8: Solutions
Section: Chapter Questions
Problem 8.10EP: A solution is made by dissolving 0.455 g of PbBr2 in 100 g of H2O at 50C. Based on the data in Table...
Related questions
Question
Transcribed Image Text:Question 30 of 30
Submit
A solution is made by dissolving 2.5 mg
sodium fluoride (NaF) in 2.5 L of water at
20.0°C. What is the fluoride ion
concentration in ppm of the resulting
solution, given that NaF is 45.0% fluoride
ion by mass, and the density of water of
1.00 kg/L?
| ppm
1
4
6.
C
7
+/-
x 10 0
Tap here or pull up for additional resources
LO
00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
