A solution is made by mixing 56.9 g of water and 23.8 g NaCl. What is the concentration of NaCl in units of percent weight/weight (% w/w)? A. 2.95% (w/w) NaCl B. 29.5% (w/w) NaCl O C. 70.5% (v/v) ethanol
A solution is made by mixing 56.9 g of water and 23.8 g NaCl. What is the concentration of NaCl in units of percent weight/weight (% w/w)? A. 2.95% (w/w) NaCl B. 29.5% (w/w) NaCl O C. 70.5% (v/v) ethanol
Chapter17: Introduction To Iv Therapy
Section: Chapter Questions
Problem 3.6P
Related questions
Question
Please answer the question
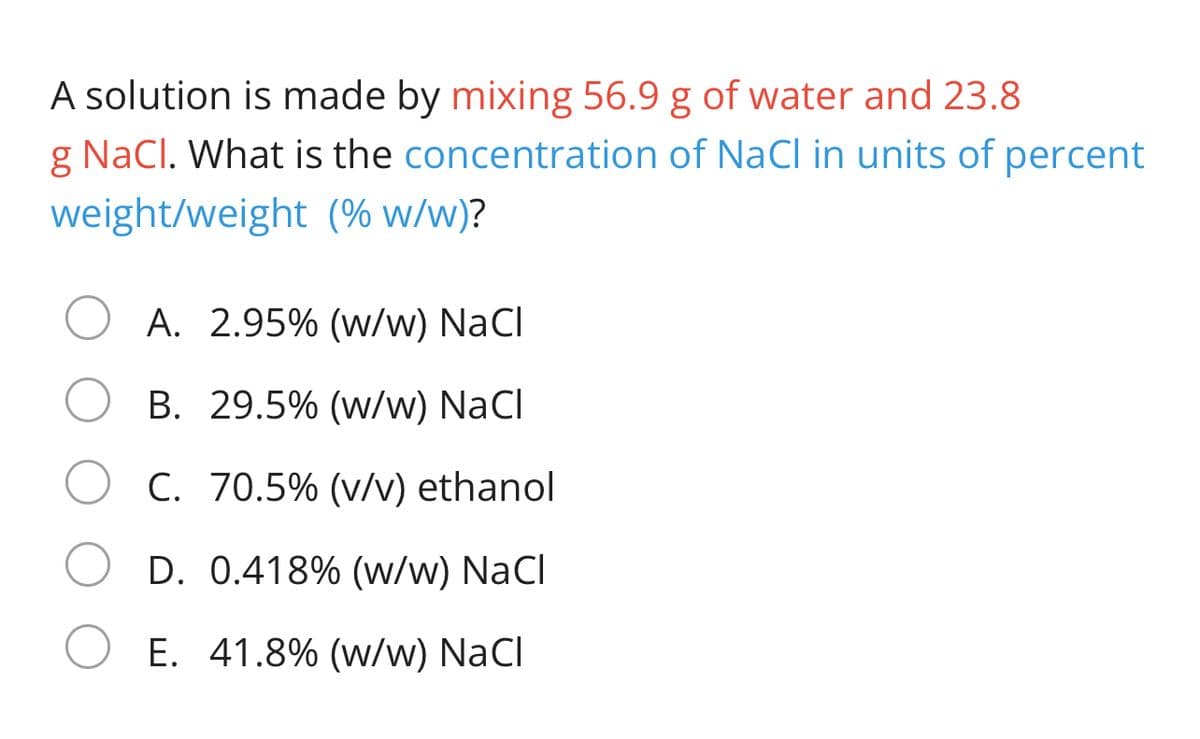
Transcribed Image Text:A solution is made by mixing 56.9 g of water and 23.8
g NaCl. What is the concentration of NaCI in units of percent
weight/weight (% w/w)?
A. 2.95% (w/w) NaCl
B. 29.5% (w/w) NaCl
C. 70.5% (v/v) ethanol
D. 0.418% (w/w) NaCl
E. 41.8% (w/w) NaCl
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you


Basic Clinical Lab Competencies for Respiratory C…
Nursing
ISBN:
9781285244662
Author:
White
Publisher:
Cengage



Basic Clinical Lab Competencies for Respiratory C…
Nursing
ISBN:
9781285244662
Author:
White
Publisher:
Cengage


