A solution is made by mixing together an aqueous solution of 1 mole of Mg(NO,), and 2 moles of aqueous NaOH. Assume the particles below each represent 1 mole of particles. Determine which ions will form a precipitate. Drag those ions into the gray box underneath the solution. Drag the left over ions from the specified mixture that do not precipitate into the blue colored solution that represents the aqueous mixture.
A solution is made by mixing together an aqueous solution of 1 mole of Mg(NO,), and 2 moles of aqueous NaOH. Assume the particles below each represent 1 mole of particles. Determine which ions will form a precipitate. Drag those ions into the gray box underneath the solution. Drag the left over ions from the specified mixture that do not precipitate into the blue colored solution that represents the aqueous mixture.
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 61E: A 100.0-mL aliquot of 0.200 M aqueous potassium hydroxide is mixed with 100.0 mL of 0.200 M aqueous...
Related questions
Question
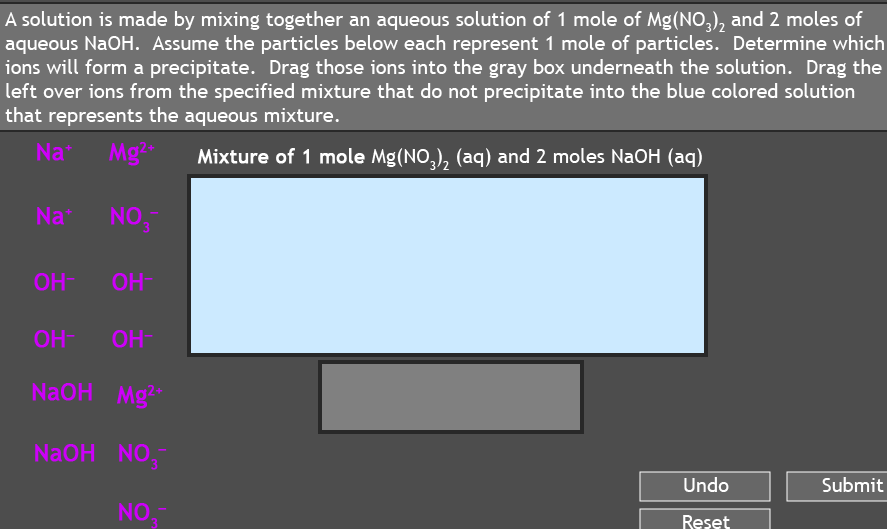
Transcribed Image Text:A solution is made by mixing together an aqueous solution of 1 mole of Mg(NO,), and 2 moles of
aqueous NaOH. Assume the particles below each represent 1 mole of particles. Determine which
ions will form a precipitate. Drag those ions into the gray box underneath the solution. Drag the
left over ions from the specified mixture that do not precipitate into the blue colored solution
that represents the aqueous mixture.
Na
Mg
Mixture of 1 mole Mg(NO,), (aq) and 2 moles NaOH (aq)
Na
NO,
OH-
OH
OH-
OH-
NaOH Mg
NaOH
NO.
Undo
Submit
NO.
Reset
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning