A solution of 0.5 M NAOH of volume 100 ml was diluted to 400 ml. What was the Molarity of the solution after dilution? O a. 0.125 M Оb. 1.22 М О с. 0.152 М O d. 1.25 M Determine the mass of Sodium produced when a 254 amp current flows for 18 hr through a cell containing molten sodium chloride. Answer:
A solution of 0.5 M NAOH of volume 100 ml was diluted to 400 ml. What was the Molarity of the solution after dilution? O a. 0.125 M Оb. 1.22 М О с. 0.152 М O d. 1.25 M Determine the mass of Sodium produced when a 254 amp current flows for 18 hr through a cell containing molten sodium chloride. Answer:
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter25: Voltammetry
Section: Chapter Questions
Problem 25.13QAP
Related questions
Question
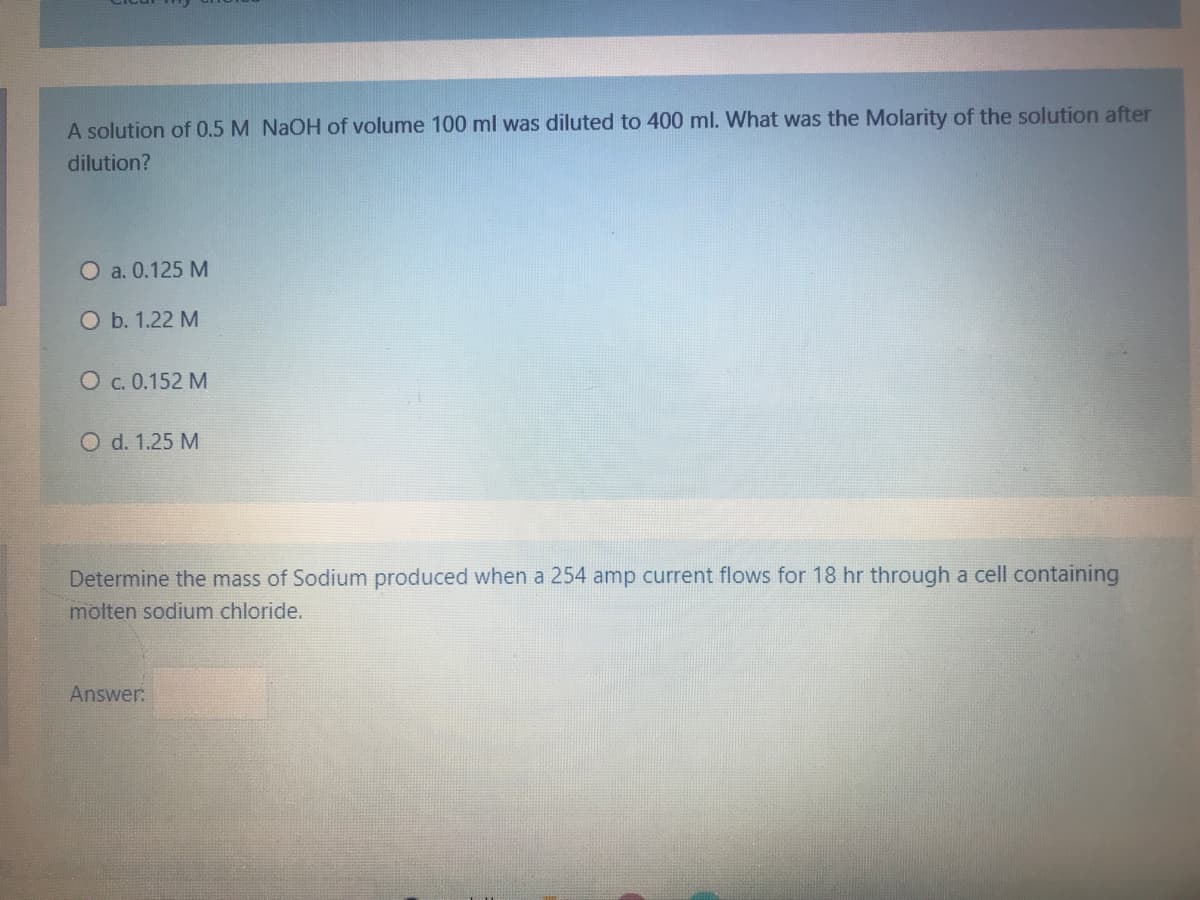
Transcribed Image Text:A solution of 0.5 M NAOH of volume 100 ml was diluted to 400 ml. What was the Molarity of the solution after
dilution?
O a. 0.125 M
O b. 1.22 M
O c. 0.152 M
O d. 1.25 M
Determine the mass of Sodium produced when a 254 amp current flows for 18 hr through a cell containing
molten sodium chloride.
Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning