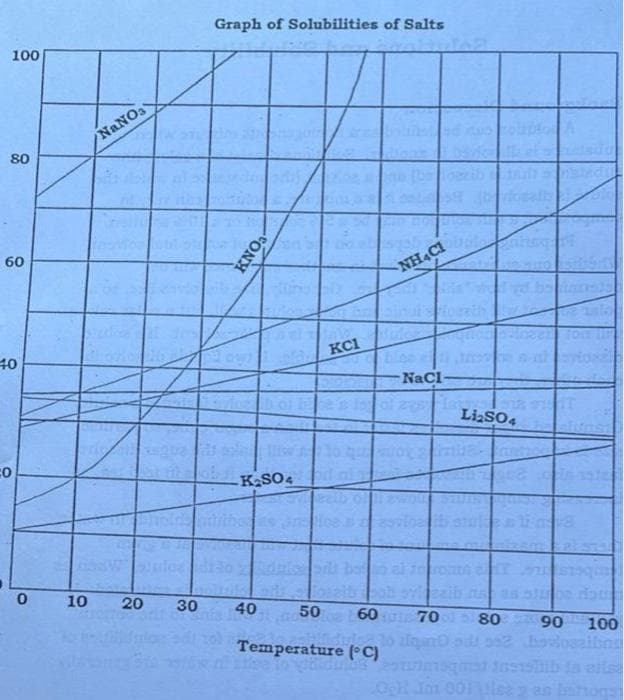
A solution saturated with NaCl and KCl at 100°C is cooled to 20° C. Assuming neither salt affects the solubility of the other, calculate the percentage NaCl and percentage KCl in the crystallized product.
A solution saturated with NaCl and KCl at 100°C is cooled to 20° C. Assuming neither salt affects the solubility of the other, calculate the percentage NaCl and percentage KCl in the crystallized product.
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter8: Solutions
Section: Chapter Questions
Problem 8.9EP: A solution is made by dissolving 34.0 g of NaCl in 100 g of H2O at 0C. Based on the data in Table...
Related questions
Question
2

Transcribed Image Text:5. A solution saturated with NaCl and KCl at 100°C is cooled to 200 C.
Assuming neither salt affects the solubility of the other, calculate
the percentage NaCl and percentage KCl in the crystallized
product.
Transcribed Image Text:100
80
60
40
CO
0
10
NaNO3
20
30
Graph of Solubilities of Salts
KNO₂
K₂SO4
40
KC1
NH4Cl
50 60
Temperature (°C)
NaCl-
Herwalt
Li₂SO4
IT
18
dou
70 80 90 100
zigun
se2 bovlosalbing
maquet toshib is ells
OgH Jm 001 lee aan
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning