E. If the statement depicts a spontaneous process write capital letter S, and if the statement does not depicts a spontaneous process write capital letter N. Write your answer on the blank provided before each item. _1. Rolling a ball uphill. _4. Waterfalls _2. Energy and entropy are both favored. _5. Water freezing at room temperature _3. Usually occurs slowly _6. Requires an energy input to proceed
E. If the statement depicts a spontaneous process write capital letter S, and if the statement does not depicts a spontaneous process write capital letter N. Write your answer on the blank provided before each item. _1. Rolling a ball uphill. _4. Waterfalls _2. Energy and entropy are both favored. _5. Water freezing at room temperature _3. Usually occurs slowly _6. Requires an energy input to proceed
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter10: Entropy And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 10.84PAE: Methane can be produced from CO and H2.The process might be done in two steps, as shown below, with...
Related questions
Question

Transcribed Image Text:E. If the statement depicts a spontaneous process write capital letter S, and if the statement does not depicts
a spontaneous process write capital letter N. Write your answer on the blank provided before each item.
_1. Rolling a ball uphill.
4. Waterfalls
2. Energy and entropy are both favored.
5. Water freezing at room
temperature
3. Usually occurs slowly
6. Requires an energy input to proceed
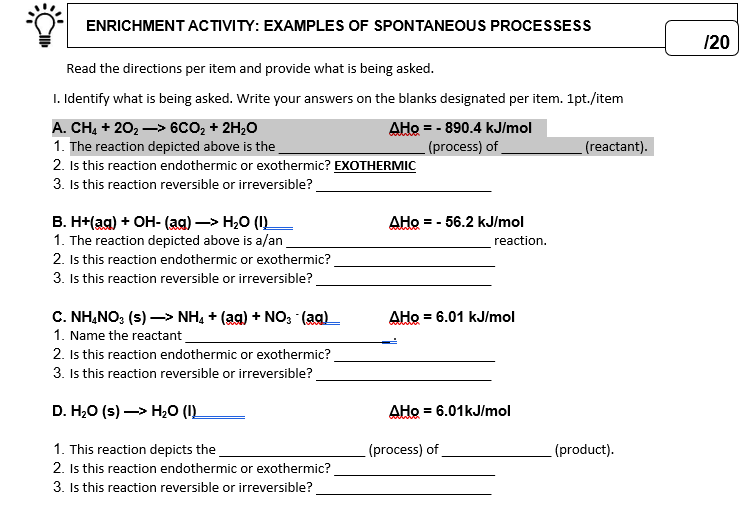
Transcribed Image Text:ENRICHMENT ACTIVITY: EXAMPLES OF SPONTANEOUS PROCESSESS
120
Read the directions per item and provide what is being asked.
I. Identify what is being asked. Write your answers on the blanks designated per item. 1pt./item
A. CH, + 202 –> 6CO2 + 2H;0
1. The reaction depicted above is the
2. Is this reaction endothermic or exothermic? EXOTHERMIC
3. Is this reaction reversible or irreversible?
AHo = - 890.4 kJ/mol
(process) of
(reactant).
В. ННая) + ОН- (aя) —> Н,О (1)
1. The reaction depicted above is a/an
2. Is this reaction endothermic or exothermic?
3. Is this reaction reversible or irreversible?
AHo = - 56.2 kJ/mol
reaction.
C. NH,NO: (s) –> NH, + (ag) + NO; (aq)
1. Name the reactant
2. Is this reaction endothermic or exothermic?
3. Is this reaction reversible or irreversible?
AHo = 6.01 kJ/mol
D. H,O (s) —> Н,0 ().
AHo = 6.01kJ/mol
1. This reaction depicts the
2. Is this reaction endothermic or exothermic?
3. Is this reaction reversible or irreversible?
(process) of
(product).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
