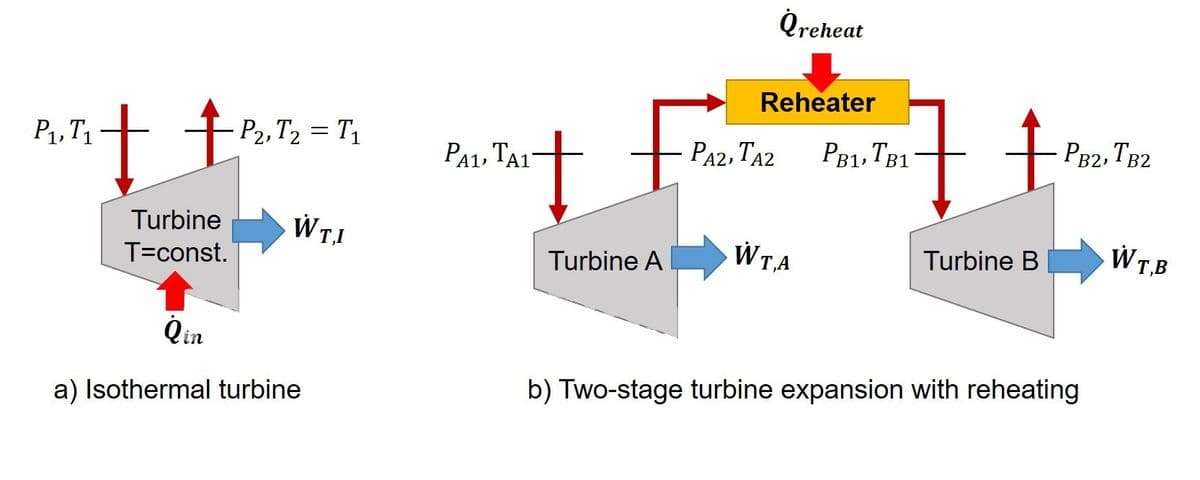
A steady-flow system operating on the ideal Ericsson cycle can achieve the Carnot efficiency. One of the salient features of this cycle is the isothermal expansion through a heated turbine, as shown in Fig. a. Heat is added to the turbine such that the outlet temperature is equal to the inlet temperature, i.e. T₁=T₂, and the overall pressure ratio for this process is rₚ=P₁/P₂. However, an isothermal expansion through a heated turbine is very difficult to achieve in practical engineering applications. An alternate approach is two use a multi-stage expansion process with reheating. In this approach, the gas expansion process is carried out in multiple stages with reheating in between each stage. Consider the two-stage turbine expansion with reheating shown in Fig. b. The gas enters the first stage of the turbine at state A1, is expanded isentropically to an intermediate pressure Pₐ₂, is reheated at constant pressure to state B1, and is expanded in the second stage isentropically to the final pressure Pᵦ₂. The overall pressure ratio remains Pₐ₁/Pᵦ₂=rₚ. In this concept, the work output is minimized if the gas is reheated to the initial temperature Tᵦ₁=Tₐ₁, and the pressure ratio is equal across each turbine stage such that Pₐ₁/Pₐ₂=Pᵦ₁/Pᵦ₂=√rₚ. For the same inlet temperature T₁=Tₐ₁, the same mass flow rate m˙, the same overall pressure ratio rₚ, and an ideal monoatomic gas as the working fluid for both scenarios. Sketch the two expansion scenarios a the T−s diagram.
A steady-flow system operating on the ideal Ericsson cycle can achieve the Carnot efficiency. One of the salient features of this cycle is the isothermal expansion through a heated turbine, as shown in Fig. a. Heat is added to the turbine such that the outlet temperature is equal to the inlet temperature, i.e. T₁=T₂, and the overall pressure ratio for this process is rₚ=P₁/P₂.
However, an isothermal expansion through a heated turbine is very difficult to achieve in practical engineering applications. An alternate approach is two use a multi-stage expansion process with reheating. In this approach, the gas expansion process is carried out in multiple stages with reheating in between each stage. Consider the two-stage turbine expansion with reheating shown in Fig. b. The gas enters the first stage of the turbine at state A1, is expanded isentropically to an intermediate pressure Pₐ₂, is reheated at constant pressure to state B1, and is expanded in the second stage isentropically to the final pressure Pᵦ₂. The overall pressure ratio remains Pₐ₁/Pᵦ₂=rₚ. In this concept, the work output is minimized if the gas is reheated to the initial temperature Tᵦ₁=Tₐ₁, and the pressure ratio is equal across each turbine stage such that Pₐ₁/Pₐ₂=Pᵦ₁/Pᵦ₂=√rₚ.
For the same inlet temperature T₁=Tₐ₁, the same mass flow rate m˙, the same overall pressure ratio rₚ, and an ideal monoatomic gas as the working fluid for both scenarios.
Sketch the two expansion scenarios a the T−s diagram.
Step by step
Solved in 2 steps with 2 images









