A stream of humid air enters a condenser in which 95% of the water sapor in the air is condensed. The flow rate of the condensation (the liquid leaving the condenser). 2254/he Dry air may be taken to contain 21 mole fu oxygen with the balance p stream nitrogens Calculate the flow rate of the gar leaving the condenser and the mole frachunt of oxygen, mitrogen, and water in this stream. 16 is the An additional of information entenns air contains 10.09midle to water prece n (1/h) 0.100 noltholio) F0,900 mst dry air/n) 0.79 mot N/moldry duf: 5 vanabler cir air -3 specier -I vol. flow rate -1952 fractant Condensade O dof hog ₁ (moto) •M₂ (mil N₂/h) n's 122521₂0(1)/2 n₂ (mult₂o/h) 22 (95% of water in feed) silve these eens below EESTILOL BUD low mot next page Sulve the See more on the equations Age on the
A stream of humid air enters a condenser in which 95% of the water sapor in the air is condensed. The flow rate of the condensation (the liquid leaving the condenser). 2254/he Dry air may be taken to contain 21 mole fu oxygen with the balance p stream nitrogens Calculate the flow rate of the gar leaving the condenser and the mole frachunt of oxygen, mitrogen, and water in this stream. 16 is the An additional of information entenns air contains 10.09midle to water prece n (1/h) 0.100 noltholio) F0,900 mst dry air/n) 0.79 mot N/moldry duf: 5 vanabler cir air -3 specier -I vol. flow rate -1952 fractant Condensade O dof hog ₁ (moto) •M₂ (mil N₂/h) n's 122521₂0(1)/2 n₂ (mult₂o/h) 22 (95% of water in feed) silve these eens below EESTILOL BUD low mot next page Sulve the See more on the equations Age on the
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Chemical engineering: solve the equations
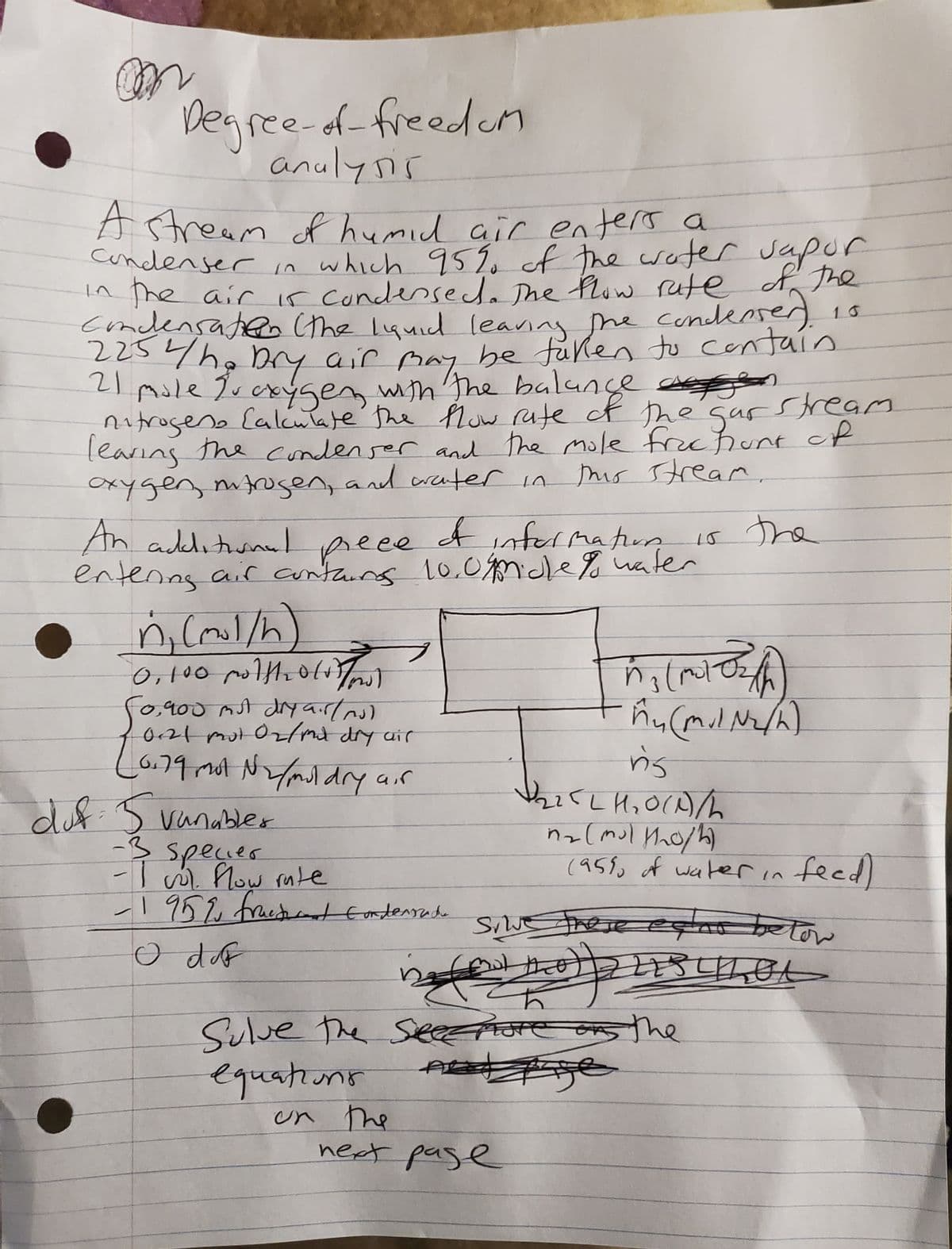
Transcribed Image Text:on
Degree-of-freedom
analysis
A stream of humid air enters a
condenser in which 95% of the water sapor
in the air is condensed. The flow rate of the
condensation (the liquid leaving the condensen), is
225 4ho Dry air may be taken to contain
21 mole Proxygen with the balance agen
nitrogens Calculate the flow rate of the gar stream
leaving the condenser and the mole frachunt of
this stream.
oxygen, nitrogen, and water in
An additional
of information
Весе
entering air contains 10.09midle to water
n₁ (mil/h)
0,100 molth2010 To
F0,900 mst dry air/no)
10121 mut 02/md dry air
(0.79 mol N₂/muldry air
duf: 5 vanabler
e
-3 specier
-I wol flow rate
-195%, fractant Condensada
O dof
equations
on the
(motor)
-M₂ (mil N₂/h)
3
n's
1₂25L1₂0(A)/
n₂ (mul M₂o/h)
Sulve the Seee more on the
AB
next page
the
(95% of water in feed)
mul tha22ESTILOL
Sive these ecent below
024

Transcribed Image Text:2
finoLA) 22521/₂000 Loogne IN IND
thou)
1,00
-3
h
18,0×10
h
15
95% condensation: 12² 0.95(0.100) (1₂)
O₂ balance: 1, (0.900), (0.21) ²/1₂
N₂ bulunce n₁ (0.900) (0.79) = (ny
H₂0 balance n, (0.100) = n₂ting
total output gar flow rate
Atotal
nge nyeng
output gar composition
n
You
YNE
in total
ny
in total
Y₁₂ J²-
ns
in total
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The