A student (Becky) is helping a friend (Taylor) solve a stoichiometry problem. Taylor needs to know how many liters of nitrogen monoxide (NO) gas are created at STP when 150 g of solid copper reacts with excess nitric acid. 3Cu(s) + 8HNO3 (aq) 3Cu(NO3), (aq) + 4H20(1) + 2NO(g) Taylor knows they should put 150 g Cu down first, and they also know they need to convert from grams to something else. Becky provides a clue to Taylor: they will need 3 conversion factors (which are empty, below). What does Taylor need in order to complete the third conversion factor? 150 g Cu 1 Coefficients from the balanced equation (molar ratio) O The molar mass of nitrogen monoxide O The molar mass of copper Avogadro's number The STP volume of gas (22.4 L/mol)
A student (Becky) is helping a friend (Taylor) solve a stoichiometry problem. Taylor needs to know how many liters of nitrogen monoxide (NO) gas are created at STP when 150 g of solid copper reacts with excess nitric acid. 3Cu(s) + 8HNO3 (aq) 3Cu(NO3), (aq) + 4H20(1) + 2NO(g) Taylor knows they should put 150 g Cu down first, and they also know they need to convert from grams to something else. Becky provides a clue to Taylor: they will need 3 conversion factors (which are empty, below). What does Taylor need in order to complete the third conversion factor? 150 g Cu 1 Coefficients from the balanced equation (molar ratio) O The molar mass of nitrogen monoxide O The molar mass of copper Avogadro's number The STP volume of gas (22.4 L/mol)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter9: Gases
Section: Chapter Questions
Problem 80E: One method of analyzing amino acids is the van Slyke method. The characteristic amino groups (NH2)...
Related questions
Question
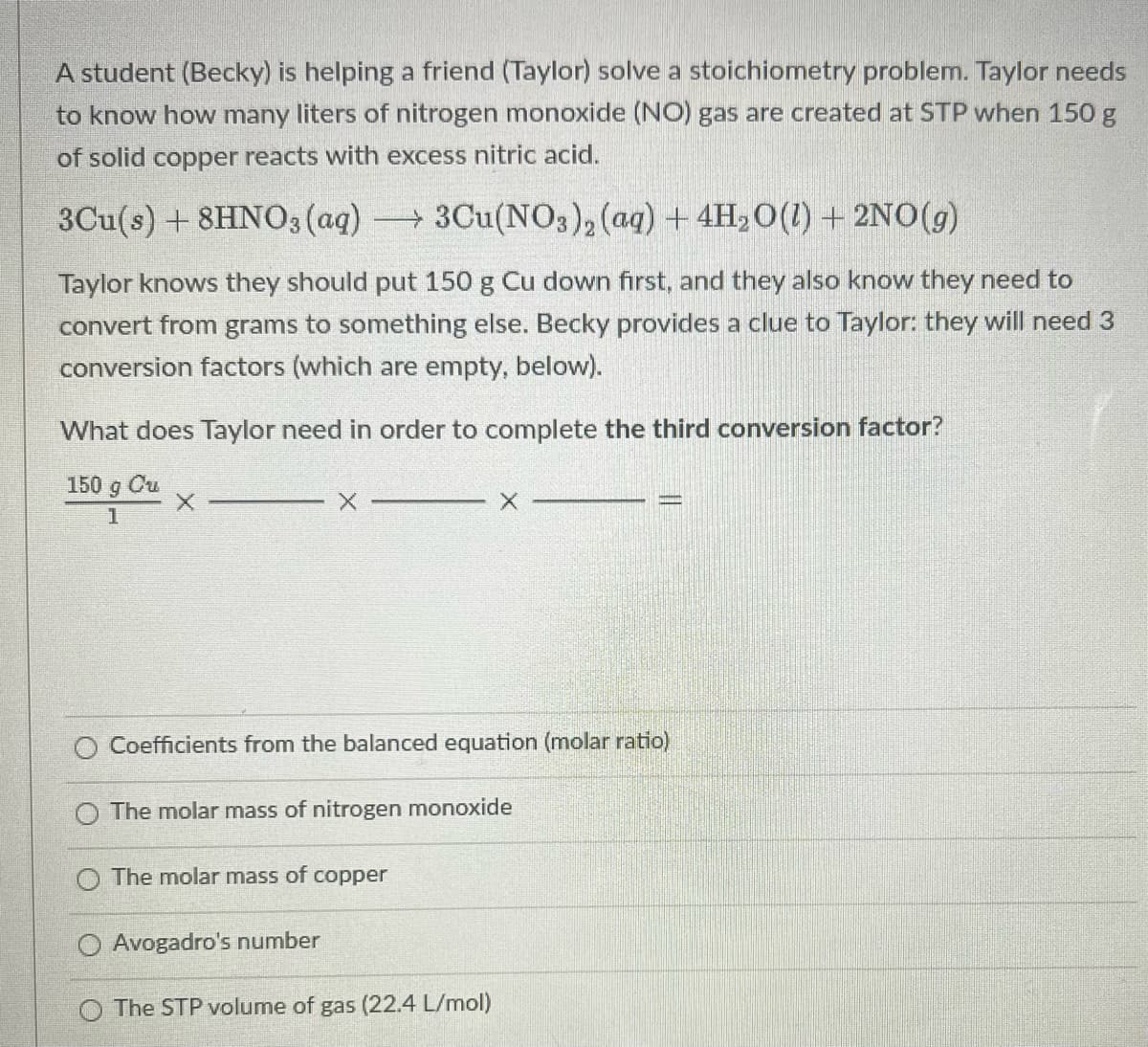
Transcribed Image Text:A student (Becky) is helping a friend (Taylor) solve a stoichiometry problem. Taylor needs
to know how many liters of nitrogen monoxide (NO) gas are created at STP when 150 g
of solid copper reacts with excess nitric acid.
3Cu(s) + 8HNO3 (aq) 3Cu(NO3), (ag) + 4H,0(1) + 2NO(g)
Taylor knows they should put 150 g Cu down first, and they also know they need to
convert from grams to something else. Becky provides a clue to Taylor: they will need 3
conversion factors (which are empty, below).
What does Taylor need in order to complete the third conversion factor?
150 g Cu
1
Coefficients from the balanced equation (molar ratio)
The molar mass of nitrogen monoxide
O The molar mass of copper
Avogadro's number
The STP volume of gas (22.4 L/mol)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning