A student claims that if element D is in group 18, then the molecular geometry of DF4(g) is square planar. Do you agree or disagree with the student? Justify your answer in terms of VSEPR theory.
A student claims that if element D is in group 18, then the molecular geometry of DF4(g) is square planar. Do you agree or disagree with the student? Justify your answer in terms of VSEPR theory.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter7: Covalent Bonding
Section: Chapter Questions
Problem 76QAP: In each of the following molecules, a central atom is surrounded by a total of three atoms or...
Related questions
Question
Hi! Please answer the question (second pic) using the graph (first pic)

Transcribed Image Text:(c) A student claims that if element D is in group 18, then the molecular geometry of DF4(g) is square planar. Do you agree or disagree with the student? Justify your answer in terms of VSEPR theory.
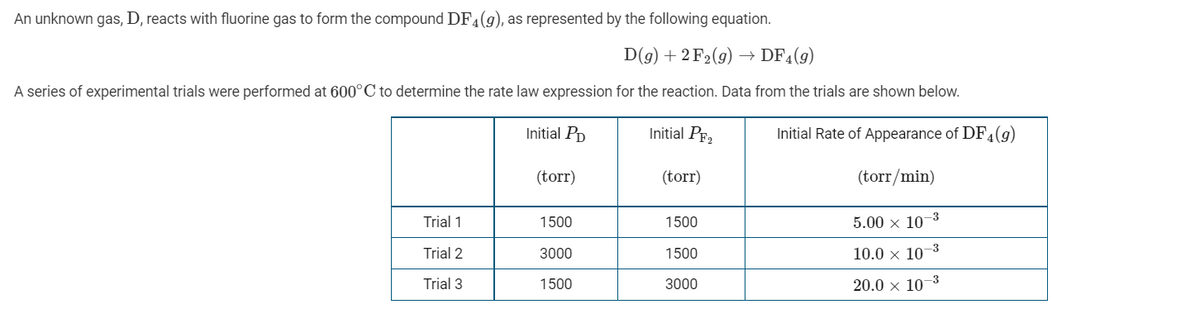
Transcribed Image Text:An unknown gas, D, reacts with fluorine gas to form the compound DF4(g), as represented by the following equation.
D(g) + 2 F2(9) → DF4(9)
A series of experimental trials were performed at 600°C to determine the rate law expression for the reaction. Data from the trials are shown below.
Initial Pp
Initial PF2
Initial Rate of Appearance of DF,(9)
(torr)
(torr)
(torr/min)
Trial 1
1500
1500
5.00 x 10–3
Trial 2
3000
1500
10.0 x 10-3
Trial 3
1500
3000
20.0 x 10–3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning