A student collected 17.14 ml of H₂ over water at 25.00 °C. The water level inside the collection apparatus was 9.5 cm higher than the water level outside. The barometric pressure was 736 torr. How many grams of zinc had to react with HCl(aq) to produce the H₂ that was collected? You may need the data in the table to solve this problem. mass= gZn Table 10.2 Vapor Pressure of Water at Various Temperatures Temperature Vapor Pressure Temperature Vapor Pressure (C) (torr) (C) (torr) 0 4.579 50 92.51 5 6.543 55. 118.04 10. 9.209 60 149.38 15 12.788 65 187,54 20 17.535 70 233,7 25 23.756 75 289.1
A student collected 17.14 ml of H₂ over water at 25.00 °C. The water level inside the collection apparatus was 9.5 cm higher than the water level outside. The barometric pressure was 736 torr. How many grams of zinc had to react with HCl(aq) to produce the H₂ that was collected? You may need the data in the table to solve this problem. mass= gZn Table 10.2 Vapor Pressure of Water at Various Temperatures Temperature Vapor Pressure Temperature Vapor Pressure (C) (torr) (C) (torr) 0 4.579 50 92.51 5 6.543 55. 118.04 10. 9.209 60 149.38 15 12.788 65 187,54 20 17.535 70 233,7 25 23.756 75 289.1
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter19: The Representative Elements
Section: Chapter Questions
Problem 92CWP
Related questions
Question
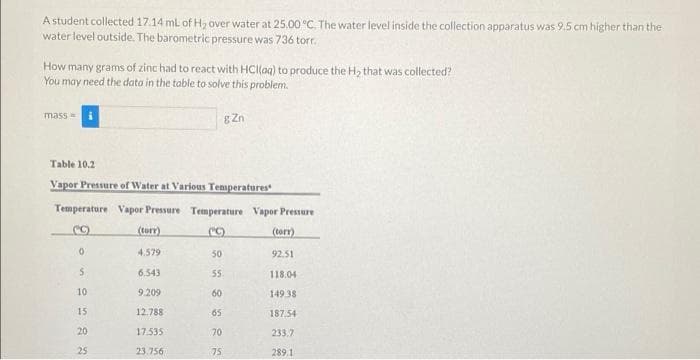
Transcribed Image Text:A student collected 17.14 ml of H₂ over water at 25.00 °C. The water level inside the collection apparatus was 9.5 cm higher than the
water level outside. The barometric pressure was 736 torr.
How many grams of zinc had to react with HCl(aq) to produce the H₂ that was collected?
You may need the data in the table to solve this problem.
mass=
gZn
Table 10.2
Vapor Pressure of Water at Various Temperatures
Temperature Vapor Pressure Temperature Vapor Pressure
(C)
(torr)
(C)
(torr)
0
4.579
50
92.51
5
6.543
55.
118.04
10.
9.209
60
149.38
15
12.788
65
187.54
20
17.535
70
233.7
25
23.756
75
289.1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
