A student made measurements on some electrochemical cells and calculated three quantities: . The standard reaction free energy AG. . The equilibrium constant K at 25.0 °C. • The cell potential under standard conditions º His results are listed below. Unfortunately, the student may have made some mistakes. Examine his results carefully and tick the box next to the incorrect quantity in each row, Note: If there is a mistake in a row, only one of the three quantities listed is wrong. Also, you may assume the number of significant digits in each qu correct. Also note: for each cell, the number n of electrons transferred per redox reaction is 2. cell A B C n 2 2 2 Explanation calculated quantities (Check the box next to any that are wrong.) AG 116. kJ/mol 62. kJ/mol -253. kJ/mol Check K O -21 4.76 × 10 O 10 7.28 x 10 O O 44 2.11 x 10 DEC O X E 0.60 V 0.32 V -1.31 V Ś O O Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Priv A tv
A student made measurements on some electrochemical cells and calculated three quantities: . The standard reaction free energy AG. . The equilibrium constant K at 25.0 °C. • The cell potential under standard conditions º His results are listed below. Unfortunately, the student may have made some mistakes. Examine his results carefully and tick the box next to the incorrect quantity in each row, Note: If there is a mistake in a row, only one of the three quantities listed is wrong. Also, you may assume the number of significant digits in each qu correct. Also note: for each cell, the number n of electrons transferred per redox reaction is 2. cell A B C n 2 2 2 Explanation calculated quantities (Check the box next to any that are wrong.) AG 116. kJ/mol 62. kJ/mol -253. kJ/mol Check K O -21 4.76 × 10 O 10 7.28 x 10 O O 44 2.11 x 10 DEC O X E 0.60 V 0.32 V -1.31 V Ś O O Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Priv A tv
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 76AP
Related questions
Question
Help with the following question
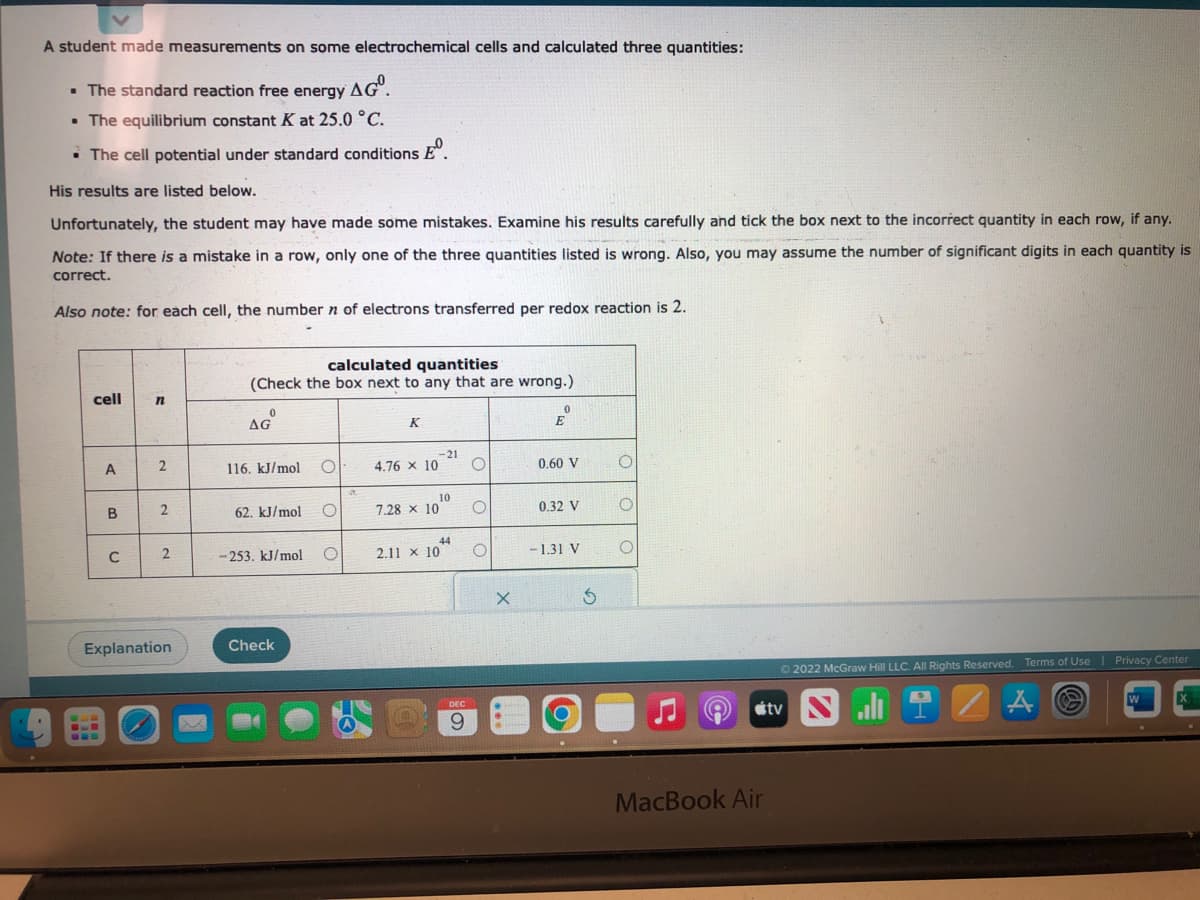
Transcribed Image Text:A student made measurements on some electrochemical cells and calculated three quantities:
. The standard reaction free energy AG.
• The equilibrium constant K at 25.0 °C.
• The cell potential under standard conditions º
His results are listed below.
Unfortunately, the student may have made some mistakes. Examine his results carefully and tick the box next to the incorrect quantity in each row, if any.
Note: If there is a mistake in a row, only one of the three quantities listed is wrong. Also, you may assume the number of significant digits in each quantity is
correct.
Also note: for each cell, the number n of electrons transferred per redox reaction is 2.
cell
A
B
C
n
2
2
2
Explanation
calculated quantities
(Check the box next to any that are wrong.)
0
AGⓇ
116. kJ/mol
62. kJ/mol
-253. kJ/mol
Check
O
O
O
A
K
4.76 × 10
7.28 x 10
-21
10
44
2.11 x 10
DEC
9
O
O
O
X
0
E
0.60 V
0.32 V
-1.31 V
Ś
O
O
O
●
MacBook Air
Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center
tv
A
W
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 8 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning