A student performed three CH₂Cl2 layer titrations in Part 2 using a standard sodium Chiosulfate solution with a concentration of 0.01155 M. They reported the following measurements: Final CH₂Cl2 Layer Burette Reading Initial CH₂Cl2 Layer Burette Reading Final Thiosulfate Burette Reading Initial Thiosulfate Burette Reading Determination #1 26.44 mL 18.67 mL 27.04 mL 0.55 mL Determination #2 33.86 mL 26.44 mL 26.18 ML 0.34 mL 4 Determination #3 41.13 mL 33.86 mL 26.07 mL 0.73 mL What is the concentration of iodine (in mol/L) in the CH₂Cl2 phase based on the data from their second titration (ie. their second determination)? Report your answer to the correct number of significant figures and only report the numerical value (no units).
A student performed three CH₂Cl2 layer titrations in Part 2 using a standard sodium Chiosulfate solution with a concentration of 0.01155 M. They reported the following measurements: Final CH₂Cl2 Layer Burette Reading Initial CH₂Cl2 Layer Burette Reading Final Thiosulfate Burette Reading Initial Thiosulfate Burette Reading Determination #1 26.44 mL 18.67 mL 27.04 mL 0.55 mL Determination #2 33.86 mL 26.44 mL 26.18 ML 0.34 mL 4 Determination #3 41.13 mL 33.86 mL 26.07 mL 0.73 mL What is the concentration of iodine (in mol/L) in the CH₂Cl2 phase based on the data from their second titration (ie. their second determination)? Report your answer to the correct number of significant figures and only report the numerical value (no units).
Chapter12: Spectrochemical Methods
Section: Chapter Questions
Problem 15P
Related questions
Question
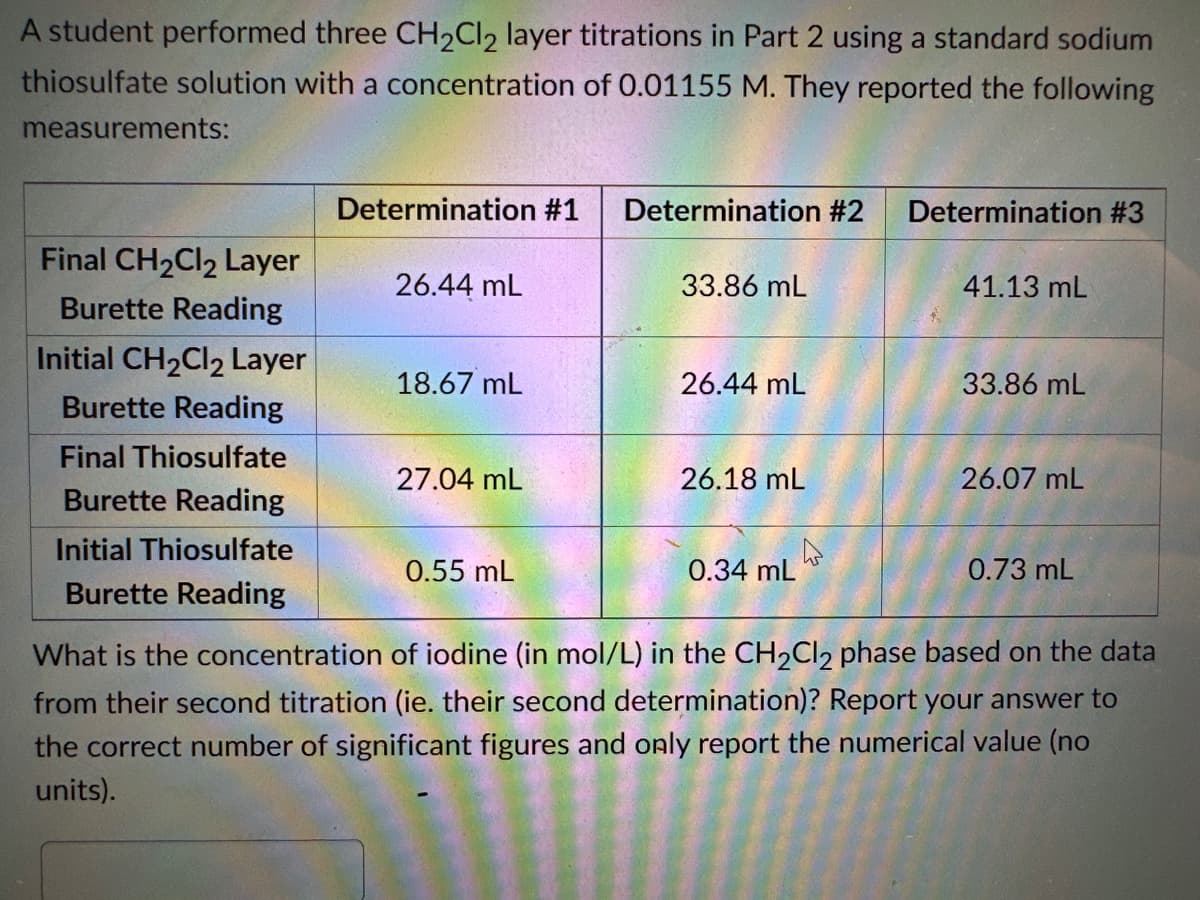
Transcribed Image Text:A student performed three CH₂Cl2 layer titrations in Part 2 using a standard sodium
thiosulfate solution with a concentration of 0.01155 M. They reported the following
measurements:
Final CH₂Cl₂ Layer
Burette Reading
Initial CH₂Cl2 Layer
Burette Reading
Final Thiosulfate
Burette Reading
Initial Thiosulfate
Burette Reading
Determination #1
26.44 mL
18.67 mL
27.04 mL
0.55 mL
Determination #2
33.86 mL
26.44 mL
26.18 ML
0.34 mL
A
Determination #3
41.13 mL
33.86 mL
26.07 mL
0.73 mL
What is the concentration of iodine (in mol/L) in the CH₂Cl2 phase based on the data
from their second titration (ie. their second determination)? Report your answer to
the correct number of significant figures and only report the numerical value (no
units).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning