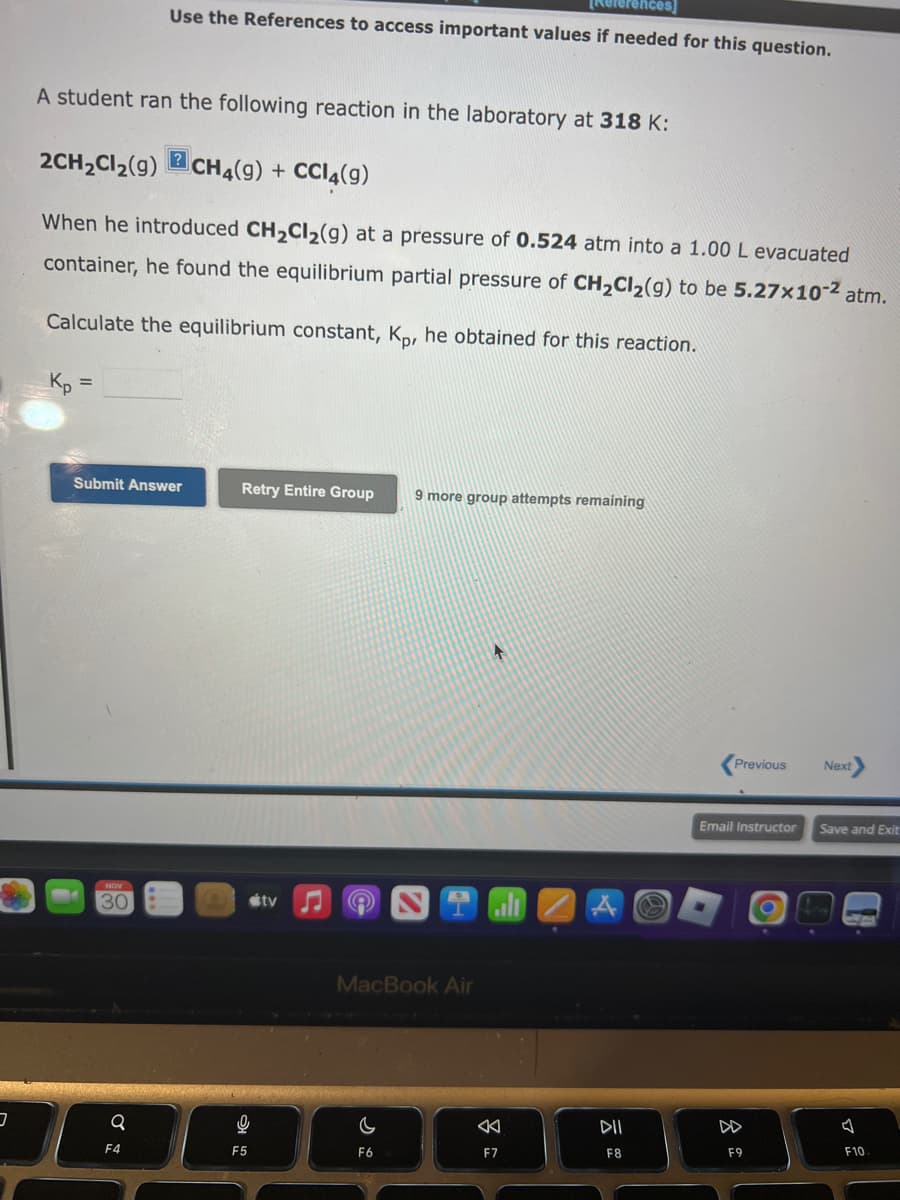
A student ran the following reaction in the laboratory at 318 K: 2CH₂Cl₂(9) CH4(9) + CCl4(9) ? es if needed for this question. When he introduced CH₂Cl₂(9) at a pressure of 0.524 atm into a 1.00 L evacuated container, he found the equilibrium partial pressure of CH₂Cl₂(g) to be 5.27x10-² atm. Calculate the equilibrium constant, Kp, he obtained for this reaction. Kp = Submit Answer Retry Entire Group 9 more group attempts remaining
A student ran the following reaction in the laboratory at 318 K: 2CH₂Cl₂(9) CH4(9) + CCl4(9) ? es if needed for this question. When he introduced CH₂Cl₂(9) at a pressure of 0.524 atm into a 1.00 L evacuated container, he found the equilibrium partial pressure of CH₂Cl₂(g) to be 5.27x10-² atm. Calculate the equilibrium constant, Kp, he obtained for this reaction. Kp = Submit Answer Retry Entire Group 9 more group attempts remaining
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter12: Gaseous Chemical Equilibrium
Section: Chapter Questions
Problem 36QAP: At a certain temperature, K=0.29 for the decomposition of two moles of iodine trichloride, ICl3(s),...
Related questions
Question
Transcribed Image Text:3
A student ran the following reaction in the laboratory at 318 K:
2CH₂Cl₂(9) CH4(9) + CCl4(9)
Use the References to access important values if needed for this question.
When he introduced CH₂Cl₂(g) at a pressure of 0.524 atm into a 1.00 L evacuated
container, he found the equilibrium partial pressure of CH₂Cl₂(g) to be 5.27x10-² atm.
Calculate the equilibrium constant, Kp, he obtained for this reaction.
Kp =
NOV
30
Submit Answer
Q
?
F4
Retry Entire Group 9 more group attempts remaining
0
F5
tv
MacBook Air
C
F6
+
F7
DII
F8
Previous Next
Email Instructor Save and Exit
F9
F10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning