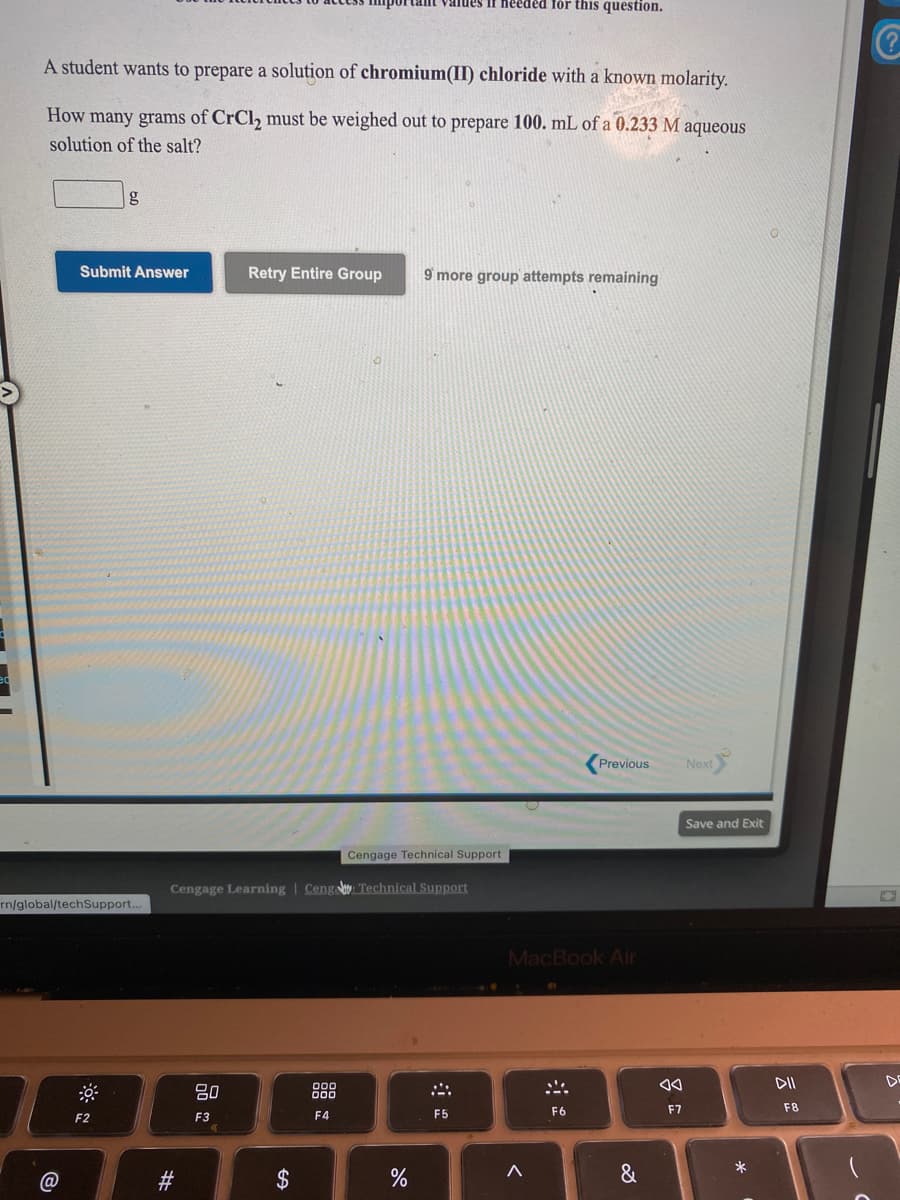
A student wants to prepare a solution of chromium(II) chloride with a known molarity. How many grams of CrCl2 must be weighed out to prepare 100. mL of a 0.233 M aqueous solution of the salt?
A student wants to prepare a solution of chromium(II) chloride with a known molarity. How many grams of CrCl2 must be weighed out to prepare 100. mL of a 0.233 M aqueous solution of the salt?
Chapter12: Gravimetric Methods Of Analysis
Section: Chapter Questions
Problem 12.31QAP
Related questions
Question
Transcribed Image Text:heeded för this question.
aiues
A student wants to prepare a solution of chromium(II) chloride with a known molarity.
How many grams of CrCl, must be weighed out to prepare 100. mL of a 0.233 M aqueous
solution of the salt?
g
Submit Answer
Retry Entire Group
9 more group attempts remaining
Previous
Next
Save and Exit
Cengage Technical Support
Cengage Learning | Cenga Technical Support
rn/global/techSupport..
MacBook Air
DII
DE
吕0
888
F6
F7
F8
F2
F3
F4
F5
@
23
&
%24
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole