A student wishes to measure the enthalpy change of the reaction of magnesium oxide solid (MgO, M= 40.30 g/mol) with hydrochloric acid (HCl, M= 36.46 g/mol), by the reaction represented in the equation below. MgO(s) + 2 HCI(ag) → MgCl,(aq) + H,0(t) (a) The student designs an experiment to dissolve 1.00g of MgO(s) in an insulated cup containing 100.g of HCI(aq) with a slight molar excess of the acid. What minimum concentration of HCI in moles per liter is needed for the experiment? Assume the density of the solution is 1.00g/mL (b) The student carries out the experiment and measures a temperature rise of 8.27°C (i) Assuming the specific heat capacity of the solution is 4.00 J/g°C, calculate the amount of heat absorbed by the solution from this reaction. (ii) Calculate the experimental enthalpy change for this reaction in KJ/moln Include the sign
A student wishes to measure the enthalpy change of the reaction of magnesium oxide solid (MgO, M= 40.30 g/mol) with hydrochloric acid (HCl, M= 36.46 g/mol), by the reaction represented in the equation below. MgO(s) + 2 HCI(ag) → MgCl,(aq) + H,0(t) (a) The student designs an experiment to dissolve 1.00g of MgO(s) in an insulated cup containing 100.g of HCI(aq) with a slight molar excess of the acid. What minimum concentration of HCI in moles per liter is needed for the experiment? Assume the density of the solution is 1.00g/mL (b) The student carries out the experiment and measures a temperature rise of 8.27°C (i) Assuming the specific heat capacity of the solution is 4.00 J/g°C, calculate the amount of heat absorbed by the solution from this reaction. (ii) Calculate the experimental enthalpy change for this reaction in KJ/moln Include the sign
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.160QP: A rebreathing gas mask contains potassium superoxide, KO2, which reacts with moisture in the breath...
Related questions
Question
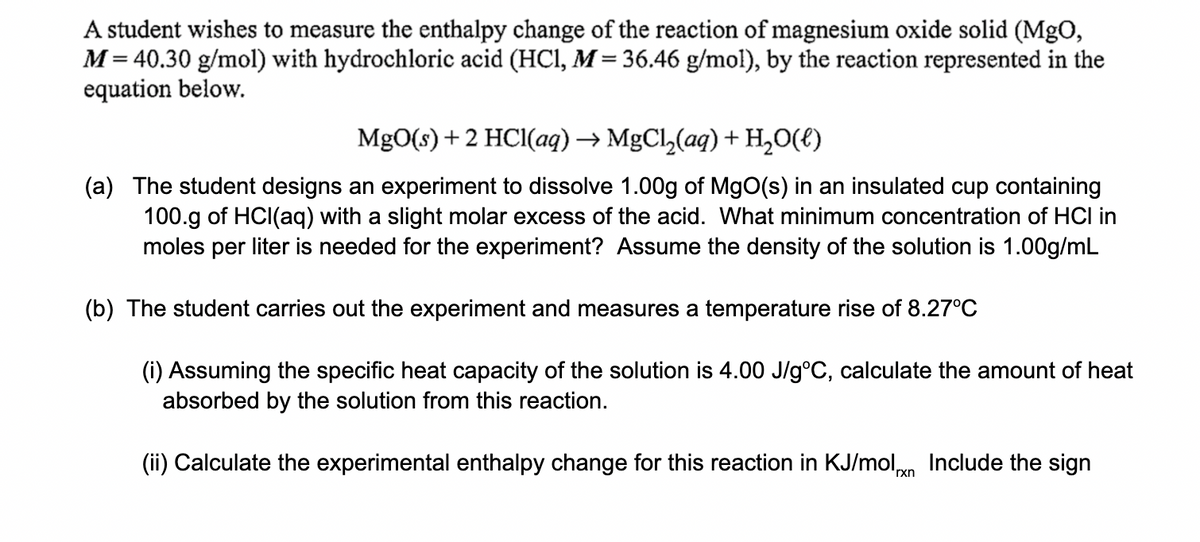
Transcribed Image Text:A student wishes to measure the enthalpy change of the reaction of magnesium oxide solid (MgO,
M= 40.30 g/mol) with hydrochloric acid (HCI, M = 36.46 g/mol), by the reaction represented in the
equation below.
MgO(s) +2 HCI(aq) → MgCl,(aq) + H,0(t)
(a) The student designs an experiment to dissolve 1.00g of MgO(s) in an insulated cup containing
100.g of HCI(aq) with a slight molar excess of the acid. What minimum concentration of HCI in
moles per liter is needed for the experiment? Assume the density of the solution is 1.00g/mL
(b) The student carries out the experiment and measures a temperature rise of 8.27°C
(i) Assuming the specific heat capacity of the solution is 4.00 J/g°C, calculate the amount of heat
absorbed by the solution from this reaction.
(ii) Calculate the experimental enthalpy change for this reaction in KJ/moln Include the sign
'rxn
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning