a system which can be assigned a value of zero at some reference point. The total energy of a system has two groups: macroscopic and microscopic. Describe the concept of macroscopic and microscopic forms of energy in a thermodynamics system. 1) A quantity of matter or a region in space chosen for study is referred to system. Some thermodynamics relations that are applicable to closed and open systems are different. It is extremely important to recognize the system before start analyzing it. Thus, differentiate among the following thermodynamics systems. (i) Isolated system; (ii) Rigid system: and
a system which can be assigned a value of zero at some reference point. The total energy of a system has two groups: macroscopic and microscopic. Describe the concept of macroscopic and microscopic forms of energy in a thermodynamics system. 1) A quantity of matter or a region in space chosen for study is referred to system. Some thermodynamics relations that are applicable to closed and open systems are different. It is extremely important to recognize the system before start analyzing it. Thus, differentiate among the following thermodynamics systems. (i) Isolated system; (ii) Rigid system: and
Automotive Technology: A Systems Approach (MindTap Course List)
6th Edition
ISBN:9781133612315
Author:Jack Erjavec, Rob Thompson
Publisher:Jack Erjavec, Rob Thompson
Chapter3: Basic Theories And Math
Section: Chapter Questions
Problem 23RQ: When applying the principles of work and force, work is accomplished when force is applied to an...
Related questions
Question
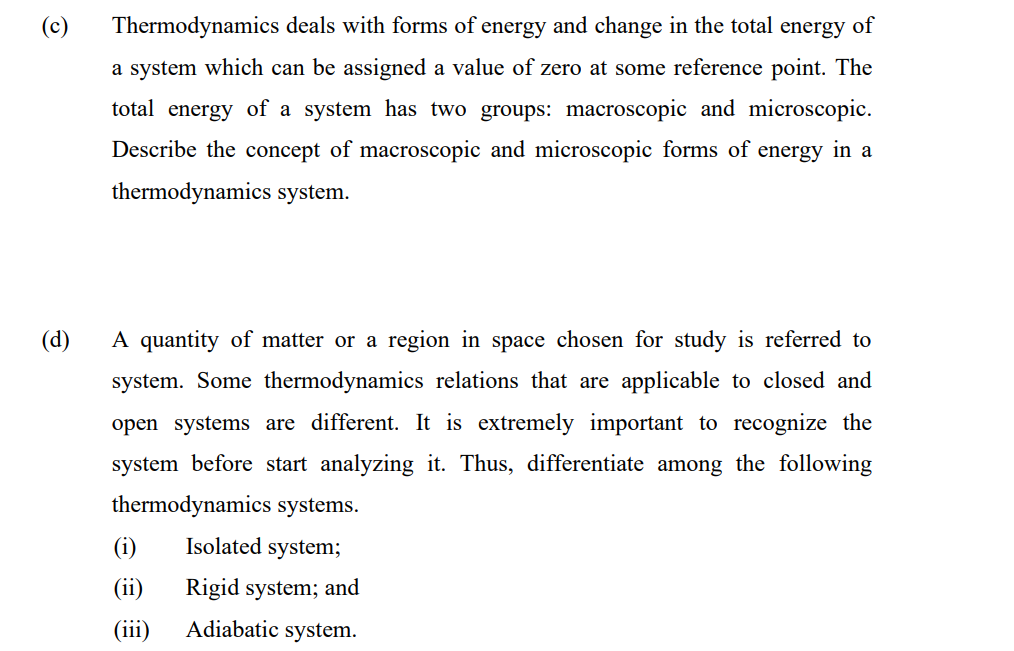
Transcribed Image Text:Thermodynamics deals with forms of energy and change in the total energy of
a system which can be assigned a value of zero at some reference point. The
total energy of a system has two groups: macroscopic and microscopic.
Describe the concept of macroscopic and microscopic forms of energy in a
thermodynamics system.
(d)
A quantity of matter or a region in space chosen for study is referred to
system. Some thermodynamics relations that are applicable to closed and
open systems are different. It is extremely important to recognize the
system before start analyzing it. Thus, differentiate among
following
thermodynamics systems.
(i)
Isolated system;
(ii)
Rigid system; and
(iii)
Adiabatic system.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Automotive Technology: A Systems Approach (MindTa…
Mechanical Engineering
ISBN:
9781133612315
Author:
Jack Erjavec, Rob Thompson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Automotive Technology: A Systems Approach (MindTa…
Mechanical Engineering
ISBN:
9781133612315
Author:
Jack Erjavec, Rob Thompson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning