M: Constaum h=chang Q2. Heat cannot be zero in closed system (True/False). * (E ce Q1. Work cannot be zero in closed system (True/False), Q3. First law of thermodynamics is not based on the law of conservation of energy (True/False), =つう-9) Q4. The change in the total energy of the closed system is Q5. The sum of the energiesCarried by the mass in open system is.Ma sshalanse Em; ={m. Q-w=AHV flnicdfrolertic.haT ehange with Time Q6. The general form of first law of thermodynamics for open system.. Q7. Un steady state flow process is defined as. internal+ Q8. Enthalpy is defined sum of kinetic and flow energy (True False). 09. Gyclic process is defined as.ha.h.ystem start from initial state trough L diffient Processes and Q10. Internal energy is considered in open system (True False)i
M: Constaum h=chang Q2. Heat cannot be zero in closed system (True/False). * (E ce Q1. Work cannot be zero in closed system (True/False), Q3. First law of thermodynamics is not based on the law of conservation of energy (True/False), =つう-9) Q4. The change in the total energy of the closed system is Q5. The sum of the energiesCarried by the mass in open system is.Ma sshalanse Em; ={m. Q-w=AHV flnicdfrolertic.haT ehange with Time Q6. The general form of first law of thermodynamics for open system.. Q7. Un steady state flow process is defined as. internal+ Q8. Enthalpy is defined sum of kinetic and flow energy (True False). 09. Gyclic process is defined as.ha.h.ystem start from initial state trough L diffient Processes and Q10. Internal energy is considered in open system (True False)i
Automotive Technology: A Systems Approach (MindTap Course List)
6th Edition
ISBN:9781133612315
Author:Jack Erjavec, Rob Thompson
Publisher:Jack Erjavec, Rob Thompson
Chapter3: Basic Theories And Math
Section: Chapter Questions
Problem 2RQ: In what four states does matter exist? Cite examples of each.
Related questions
Question
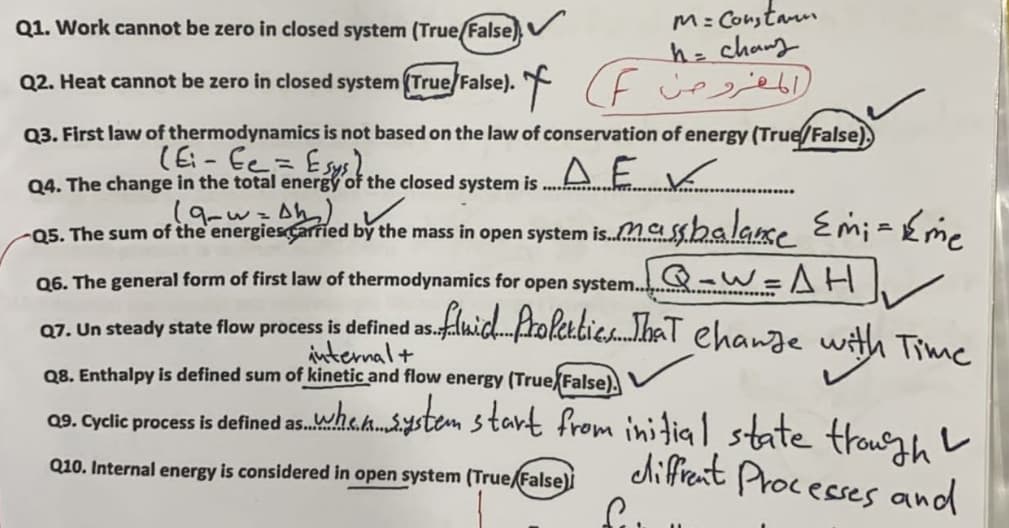
Transcribed Image Text:M: Constarm
h=chang
Q2. Heat cannot be zero in closed system (True/False). ( F cee
Q1. Work cannot be zero in closed system (True/False)
Q3. First law of thermodynamics is not based on the law of conservation of energy (True/False),
Q4. The change in the total energy of the closed system is
-Q5. The sum of the'energiesCarried by the mass in open system is.Mashalase Emi =Eme
Q-w=AH
fluichProleabie.haT ehange with Time
Q6. The general form of first law of thermodynamics for open system..
Q7. Un steady state flow process is defined as.
internal+
Q8. Enthalpy is defined sum of kinetic and flow energy (True False),
09. Cyclic process is defined as.wha.h.ystem start from initial state trour, L
diffrent Processes and
.e.k...
Q10. Internal energy is considered in open system (True/False)İ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Automotive Technology: A Systems Approach (MindTa…
Mechanical Engineering
ISBN:
9781133612315
Author:
Jack Erjavec, Rob Thompson
Publisher:
Cengage Learning

Automotive Technology: A Systems Approach (MindTa…
Mechanical Engineering
ISBN:
9781133612315
Author:
Jack Erjavec, Rob Thompson
Publisher:
Cengage Learning