A tank containing potable water is under an absolute pressure of 60 KPa and has a temperature of 600C. Determine the mass of the air in the tank. Use Fig.1.
A tank containing potable water is under an absolute pressure of 60 KPa and has a temperature of 600C. Determine the mass of the air in the tank. Use Fig.1.
International Edition---engineering Mechanics: Statics, 4th Edition
4th Edition
ISBN:9781305501607
Author:Andrew Pytel And Jaan Kiusalaas
Publisher:Andrew Pytel And Jaan Kiusalaas
Chapter8: Centroids And Distributed Loads
Section: Chapter Questions
Problem 8.121P: One side of the container has a 03-m square door that is hinged at its top edge. If the container is...
Related questions
Question
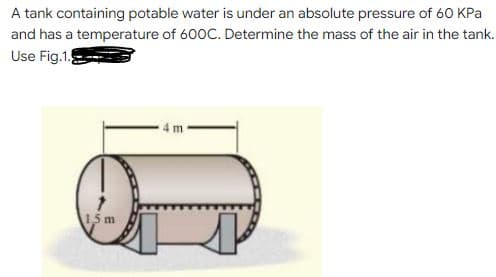
Transcribed Image Text:A tank containing potable water is under an absolute pressure of 60 KPa
and has a temperature of 600C. Determine the mass of the air in the tank.
Use Fig.1.
15 m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

International Edition---engineering Mechanics: St…
Mechanical Engineering
ISBN:
9781305501607
Author:
Andrew Pytel And Jaan Kiusalaas
Publisher:
CENGAGE L

International Edition---engineering Mechanics: St…
Mechanical Engineering
ISBN:
9781305501607
Author:
Andrew Pytel And Jaan Kiusalaas
Publisher:
CENGAGE L