(a) The BCC iron has a density of 7882 kg/m³ and the lattice parameter of 0.287 nm when hydrogen atoms are introduced at interstitial positions. Evaluate (i) the atomic fraction of hydrogen atoms (ii) the number of unit cells on average that contain hydrogen atoms (b) The strength values of a tungsten-tantalum alloy and its related properties are shown in the table below: Grain diameter (mm) Strength (MPa) 0.071 140 0.110 134 0.203 129 0.314 126 Determine (i) the constants in the Hall-Petch equation. (ii) the grain size required to obtain strength of 300 MPa.
(a) The BCC iron has a density of 7882 kg/m³ and the lattice parameter of 0.287 nm when hydrogen atoms are introduced at interstitial positions. Evaluate (i) the atomic fraction of hydrogen atoms (ii) the number of unit cells on average that contain hydrogen atoms (b) The strength values of a tungsten-tantalum alloy and its related properties are shown in the table below: Grain diameter (mm) Strength (MPa) 0.071 140 0.110 134 0.203 129 0.314 126 Determine (i) the constants in the Hall-Petch equation. (ii) the grain size required to obtain strength of 300 MPa.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter22: Inorganic Materials
Section: Chapter Questions
Problem 9P
Related questions
Question
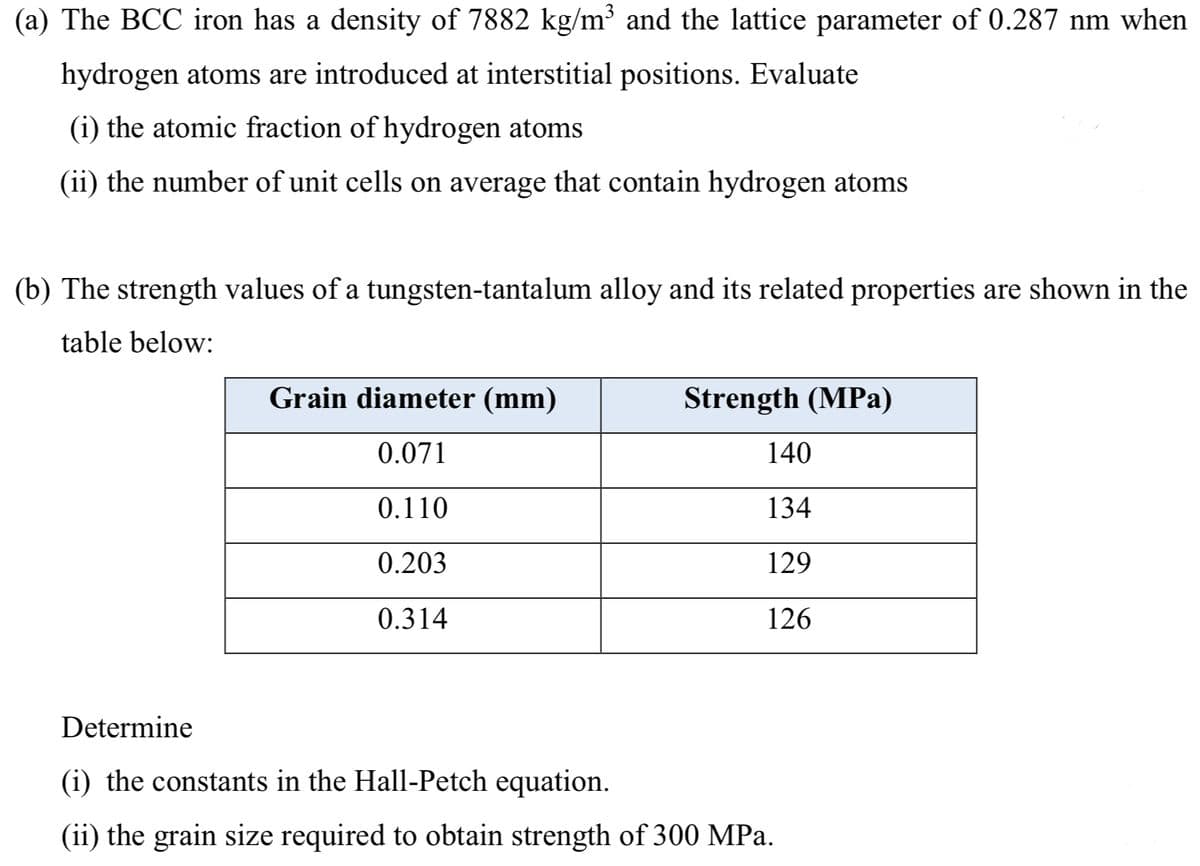
Transcribed Image Text:(a) The BCC iron has a density of 7882 kg/m³ and the lattice parameter of 0.287 nm when
hydrogen atoms are introduced at interstitial positions. Evaluate
(i) the atomic fraction of hydrogen atoms
(ii) the number of unit cells on average that contain hydrogen atoms
(b) The strength values of a tungsten-tantalum alloy and its related properties are shown in the
table below:
Grain diameter (mm)
Strength (MPa)
0.071
140
0.110
134
0.203
129
0.314
126
Determine
(i) the constants in the Hall-Petch equation.
(ii) the grain size required to obtain strength of 300 MPa.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning