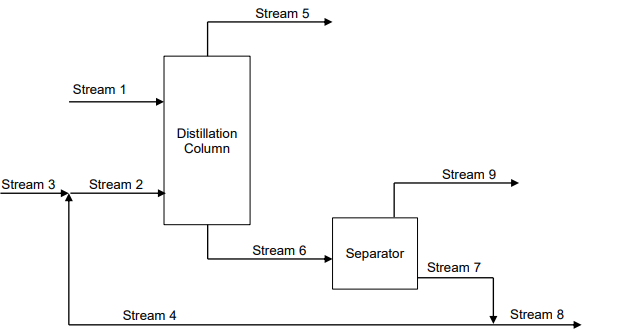
A two-unit separation is used to dewater ethanol. First, a 320 mol/hr feed (Stream 1) of a mixture of 0.750 mole fraction ethanol (E) and 0.250 mole fraction water (W) is fed to a distillation column. The extractive agent, benzene (B), is added to the distillation column in a mixture of 0.940 mole fraction benzene and 0.060 mole fraction water (Stream2). This mixture consists of fresh benzene (Stream 3) and the recycle stream of benzene and water recovered from the separator (Stream 4). The company wishes to produce a 0.98 mole fraction ethanol (0.02 mole fraction benzene) product stream from the overhead of the distillation column (Stream 5). The bottoms product (Stream 6), containing just benzene and water, is sent to the separator. The composition of the benzene/water mixture leaving the bottom of the separator (Stream 7) is 0.86 mole fraction benzene and the balance water. Some of the bottom stream leaving the separator is recycled (Stream 4) and added to the fresh benzene feed to the column and the rest of the bottom stream (57 mol/hr) is sent to another unit (Stream 8). The stream leaving the top of the separator (Stream 9) is pure water. a) Why is a recycle stream (Stream 4) included in this process? b) What is the molar flowrate of the ethanol product (Stream 5)? c) What is the molar flowrate of water removed in the separator (Stream 9)? d) What is the molar flowrate of fresh benzene (Stream 3)? e) What are the molar flowrate and mole fractions of the distillation column bottoms stream 6
A two-unit separation is used to dewater ethanol. First, a 320 mol/hr feed
(Stream 1) of a mixture of 0.750 mole fraction ethanol (E) and 0.250 mole
fraction water (W) is fed to a distillation column. The extractive agent, benzene
(B), is added to the distillation column in a mixture of 0.940 mole fraction
benzene and 0.060 mole fraction water (Stream2). This mixture consists of
fresh benzene (Stream 3) and the recycle stream of benzene and water
recovered from the separator (Stream 4). The company wishes to produce a
0.98 mole fraction ethanol (0.02 mole fraction benzene) product stream from
the overhead of the distillation column (Stream 5). The bottoms product
(Stream 6), containing just benzene and water, is sent to the separator. The
composition of the benzene/water mixture leaving the bottom of the separator
(Stream 7) is 0.86 mole fraction benzene and the balance water. Some of the
bottom stream leaving the separator is recycled (Stream 4) and added to the
fresh benzene feed to the column and the rest of the bottom stream (57 mol/hr)
is sent to another unit (Stream 8). The stream leaving the top of the separator
(Stream 9) is pure water.
a) Why is a recycle stream (Stream 4) included in this process?
b) What is the molar flowrate of the ethanol product (Stream 5)?
c) What is the molar flowrate of water removed in the separator (Stream 9)?
d) What is the molar flowrate of fresh benzene (Stream 3)?
e) What are the molar flowrate and mole fractions of the distillation column bottoms stream 6
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 1 images









