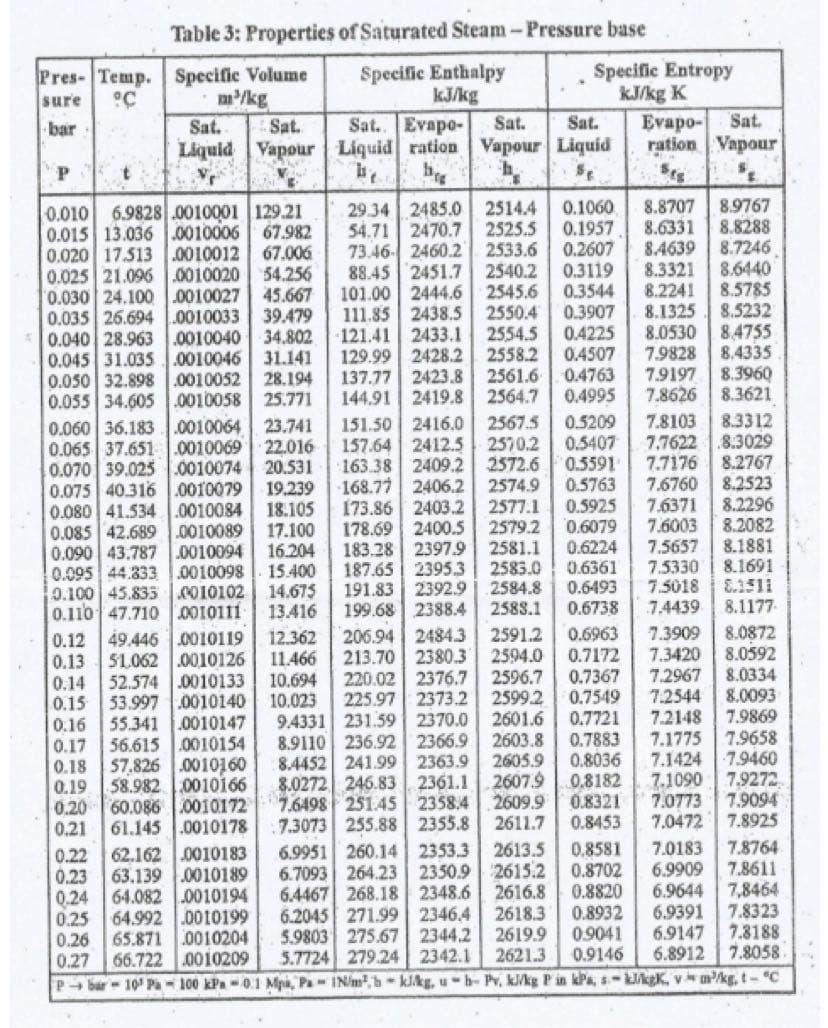
Using Steam Table, find Ahf and Ahg in BTU/lbm and Asf and Asg in BTU/lbm-°R for the following parameters: 1. P₁ = 86.6 kPa; P2 = 101.3 kPa 2. T₁ = 90°F; T₂ = 125°F 3. P₁ = 4.7 psi; P2 = 12 psi
Using Steam Table, find Ahf and Ahg in BTU/lbm and Asf and Asg in BTU/lbm-°R for the following parameters: 1. P₁ = 86.6 kPa; P2 = 101.3 kPa 2. T₁ = 90°F; T₂ = 125°F 3. P₁ = 4.7 psi; P2 = 12 psi
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Transcribed Image Text:Pres-Temp.
sure °C
bar
P
t
Table 3: Properties of Saturated Steam-Pressure base
Specific Volume
Specific Enthalpy
m³/kg
kJ/kg
Sat.
Sat.
Liquid Vapour
67.982
67.006
54.256
45.667
0.010 6.9828 .0010001 129.21
0.015 13.036 .0010006
0.020 17.513 .0010012
0.025 21.096 0010020
0.030 24.100 .0010027
39.479
0.035 26.694.0010033
0.040 28.963.0010040 34.802
0.045 31.035 .0010046 31.141
0.050 32.898 0010052 28.194
0.055 34.605 0010058 25.771
0.060 36.183 0010064 23.741
0.065 37.651 0010069 22.016
0.070 39.025 .0010074 20.531
19,239
0.075 40.316 0010079
0.080 41.534 .0010084
18.105
17.100
0.085 42.689 0010089
0.090 43.787 0010094
16.204
15.400
0.095 44.333 0010098
14.675
0.100 45.835 0010102
0.110 47.7100010111 13.416
0.14
0.15
0.12 49.446 0010119 12.362
51.062 0010126 11.466
0.13
0.16 55.341 0010147
0.17 56.615 0010154
0.18 57.826 0010160
0.19 58.982 0010166
0.20 60.086 0010172
0.21
61.145 0010178
Sat. | Evape-
Sat.
Sat.
Liquid ration Vapour Liquid
I
St
Specific Entropy
kJ/kg K
183.28 2397.9
2581.1
187.65 2395.3 2583.0
191.83 2392.9
199.68 2388.4
206.94 2484.3
213.70 2380.3
29.34 2485.0 2514.4
54.71 2470.7 2525.5
73.46 2460.2 2533.6
88.45 2451.7 2540.2
101.00 2444.6 2545.6
111.85 2438.5
121.41 2433.1
129.99 2428.2
137.77 2423.8
144.91 2419.8
151.50 2416.0
2567.5
0.5209 7.8103
157.64 2412.5 2570.2 0.5407 7.7622
163.38 2409.2 2572.6 0.5591 7.717
168.77 2406.2 2574.9 0.5763
7.6760
173.86 2403.2
2577.1
0.5925
7.6371
178.69 2400.5
2579.2
0.6079
7.6003
0.6224
7.5657
0.6361
7.5330
0.6493 7.5018
2584.8
2583.1 0.6738 7.4439
2591.2 0.6963 7.3909
0.7172
2594.0
7.3420
2596.7 0.7367
7.2967
8.0334
0.7549
7.2544
8.0093
7.2148
7.9869
7.1775
7.9658
7.1424 -7.9460
7.1090
7.0773
7.0472
Evapo-
Sat.
ration Vapour
0.1060
0.1957
0.2607
0.3119
0.3544
2550.4 0.3907
2554.5
0.4225
2558.2
0.4507 7.9828
2561.6-
0.4763 7.9197
2564.7
0.4995 7.8626
8.8707
8.6331
8.4639
8.3321
8.2241
8.1325
8.0530
0.7721
2603.8 0.7883
2605.9 0.8036
2607.9 0.8182
2609.9 0.8321
2611.7
0.8453
8.9767
8.8288
8.7246
8.6440
8.5785
8.5232
8.4755
8.4335
8.3960
8.3621
220.02 2376.7
225.97 2373.2 2599.2
2601.6
10.694
52.574 0010133
53.997 0010140 10.023
9.4331 231.59 2370.0
8.9110 236.92 2366.9
8.4452 241.99 2363.9
8.0272 246.83 2361.1
7.6498 251.45 2358.4
2355.8
6.9951 260.14 2353.3 2613.5
7.3073 255.88
0.22
62.162 0010183
0.8581 7.0183
7.8764
0.23
63.139
0.8702
6.9909
7.8611
2616.8
0.8820
6.9644 7,8464
2618.3
0.8932
6.9391 7.8323
189 6.7093 264.23 2350.9 2615.2
6.4467 268.18 2348.6
0.24 64.082 0010194
6.2045 271.99 2346.4
0.25 64.992 0010199
0.26 65.871 0010204 5.9803 275.67 2344.2
5.7724 279.24 2342.1
0.27
2621.3 0.9146 6.8912
66.722 0010209
Pbar-10¹ Pa-100 kPa -0.1 Mpa, Pa IN/m²,b-kikg, u-b- Pv, kJ/kg P in kPa, s-kl/kgK, vmkg, t-C
2619.9 0.9041
6.9147
8.3312
8.3029
8.2767
8.2523
8.2296
8.2082
8.1881
8.1691
6.1511
8.1177
8.0872
8.0592
7.9272
7.9094
7.8925
7.8188
7.8058

Transcribed Image Text:Using Steam Table, find Ahf and Ahg in
BTU/lbm and Asf and Asg in BTU/lbm-°R for
the following parameters:
1. P₁ = 86.6 kPa; P₂ = 101.3 kPa
2. T₁ = 90°F; T2 = 125°F
3. P₁ = 4.7 psi; P2 = 12 psi
Expert Solution
Step by step
Solved in 9 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The