A volume of 100 mL of 1.00 M HCl solution is titrated with 1.00 M NaOH solution. You added the following quantities of 1.00 M NaOH to ther flask. Classify the following conditions based on whether they are before the equivalence point, at the equivalence point, or after the equivalence poi Drag the appropriate items to their respective bins. • View Available Hint(s) Reset Hel 150 mL of 1.00 M NaOH 10.0 mL of 1.00 M NAOH 5.00 mL of 1.00 M NaOH 100 mL of 1.00 M NaOH 200 mL of 1.00 M NaOH 50.0 mL of 1.00 M NaOH Before equivalence point At equivalence point After equivalence point
A volume of 100 mL of 1.00 M HCl solution is titrated with 1.00 M NaOH solution. You added the following quantities of 1.00 M NaOH to ther flask. Classify the following conditions based on whether they are before the equivalence point, at the equivalence point, or after the equivalence poi Drag the appropriate items to their respective bins. • View Available Hint(s) Reset Hel 150 mL of 1.00 M NaOH 10.0 mL of 1.00 M NAOH 5.00 mL of 1.00 M NaOH 100 mL of 1.00 M NaOH 200 mL of 1.00 M NaOH 50.0 mL of 1.00 M NaOH Before equivalence point At equivalence point After equivalence point
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 14.97QE: According to the Resource Conservation and Recovery Act (RCRA), waste material is classified as...
Related questions
Question
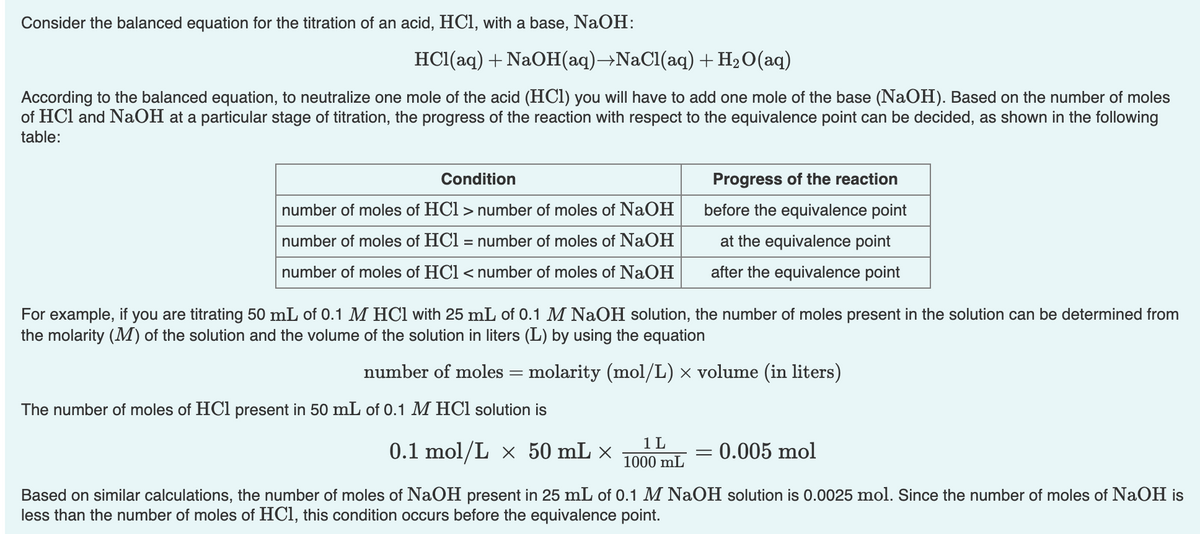
Transcribed Image Text:Consider the balanced equation for the titration of an acid, HCl, with a base, NaOH:
HC1(aq) + NaOH(aq)→NaCl(aq) + H2O(aq)
According to the balanced equation, to neutralize one mole of the acid (HCl) you will have to add one mole of the base (NaOH). Based on the number of moles
of HCl and NaOH at a particular stage of titration, the progress of the reaction with respect to the equivalence point can be decided, as shown in the following
table:
Condition
Progress of the reaction
number of moles of HCl > number of moles of NaOH
before the equivalence point
number of moles of HCl = number of moles of NaOH
at the equivalence point
number of moles of HCl < number of moles of NaOH
after the equivalence point
For example, if you are titrating 50 mL of 0.1 M HCl with 25 mL of 0.1 M NaOH solution, the number of moles present in the solution can be determined from
the molarity (M) of the solution and the volume of the solution in liters (L) by using the equation
number of moles = molarity (mol/L) × volume (in liters)
The number of moles of HCl present in 50 mL of 0.1 M HCl solution is
0.1 mol/L × 50 mL ×
1 L
1000 mL
0.005 mol
Based on similar calculations, the number of moles of NaOH present in 25 mL of 0.1 M NaOH solution is 0.0025 mol. Since the number of moles of NaOH is
less than the number of moles of HCl, this condition occurs before the equivalence point.
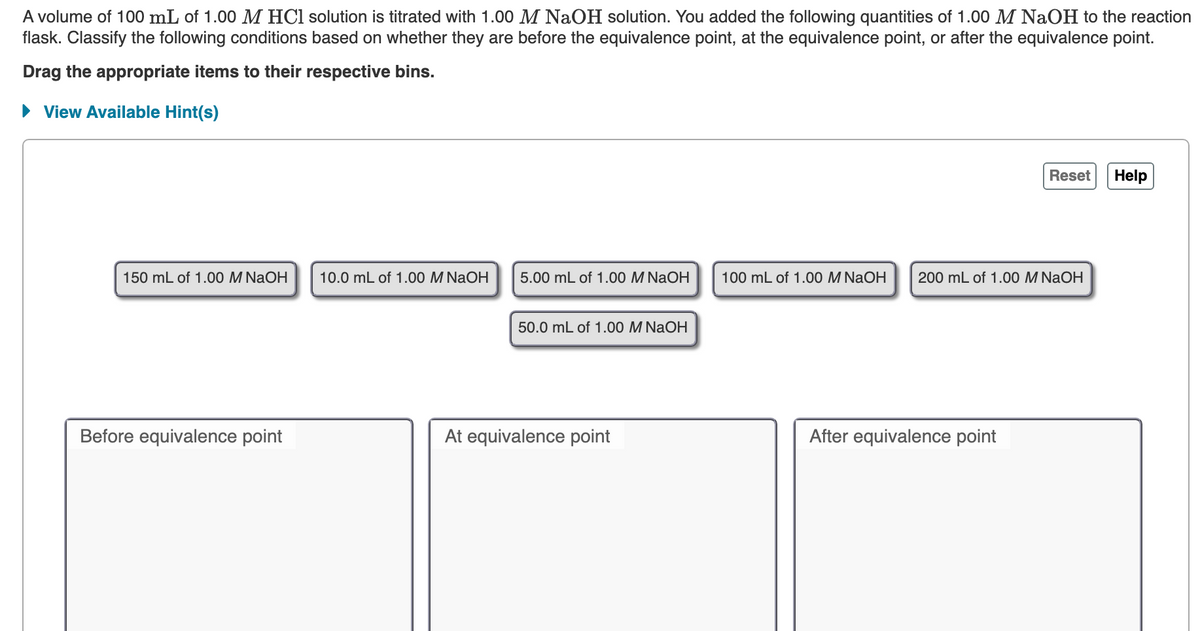
Transcribed Image Text:A volume of 100 mL of 1.00 M HCl solution is titrated with 1.00 M NaOH solution. You added the following quantities of 1.00 M NaOH to the reaction
flask. Classify the following conditions based on whether they are before the equivalence point, at the equivalence point, or after the equivalence point.
Drag the appropriate items to their respective bins.
• View Available Hint(s)
Reset
Help
150 mL of 1.00 M NaOH
10.0 mL of 1.00 M NaOH
5.00 mL of 1.00 M NaOH
100 mL of 1.00 M NaOH
200 mL of 1.00 M NaOH
50.0 mL of 1.00 M NaOH
Before equivalence point
At equivalence point
After equivalence point
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning