A volume of 80.0 mL of H20 is initially at room temperature (22.00 °C). A chilled steel rod at 2.00 °Cis placed in the water. If the final temperature of the system is 21.50 °C, what is the mass of the steel bar? Use the following values: specific heat of water = 4.18 J/(g - °C) specific heat of steel = 0.452 J/(g - °C) Express your answer to three significant figures and include the appropriate units.
A volume of 80.0 mL of H20 is initially at room temperature (22.00 °C). A chilled steel rod at 2.00 °Cis placed in the water. If the final temperature of the system is 21.50 °C, what is the mass of the steel bar? Use the following values: specific heat of water = 4.18 J/(g - °C) specific heat of steel = 0.452 J/(g - °C) Express your answer to three significant figures and include the appropriate units.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.94PAE
Related questions
Question
100%
Transcribed Image Text:I Review | Constants | Periodic Table
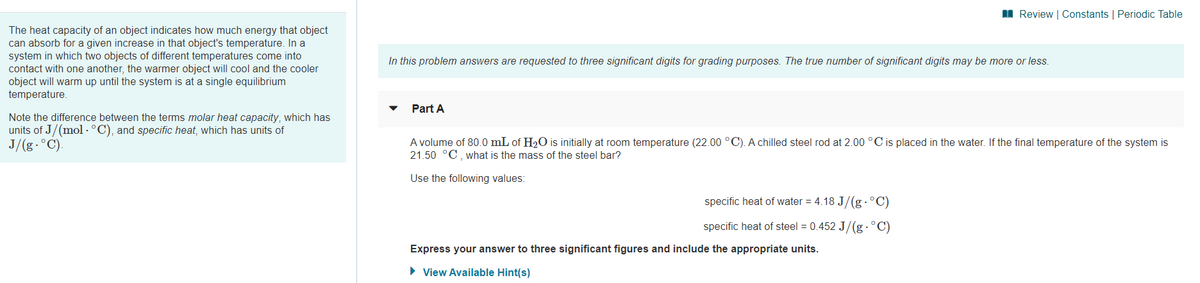
The heat capacity of an object indicates how much energy that object
can absorb for a given increase in that object's temperature. In a
system in which two objects of different temperatures come into
contact with one another, the warmer object will cool and the cooler
object will warm up until the system is at a single equilibrium
temperature.
In this problem answers are requested to three significant digits for grading purposes. The true number of significant digits may be more or less.
Part A
Note the difference between the terms molar heat capacity, which has
units of J/(mol · °C), and specific heat, which has units of
J/(g.°C).
A volume of 80.0 mL of H2O is initially at room temperature (22.00 °C). A chilled steel rod at 2.00 °C is placed in the water. If the final temperature of the system is
21.50 °C, what is the mass of the steel bar?
Use the following values:
specific heat of water = 4.18 J/(g .°C)
specific heat of steel = 0.452 J/(g .°C)
Express your answer to three significant figures and include the appropriate units.
• View Available Hint(s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning