(a) What is the best coefficient of performance for a refrigerator that cools an environment at -28.5°C and has heat transfer to another environment at 46.5°C? 3.262 (b) How much work in joules must be done for a heat transfer of 4186 k) from the cold environment? 1283.26 (c) What is the cost (in cents) of doing this if the work costs 15.0 cents per 3.60 x 106 J (a kilowatt-hour)? 5.35 (d) How many k) of heat transfer occurs into the warm environment? 5469 26 v k) (e) Discuss what type of refrigerator might operate between these temperatures. The inside of the refrigerator (actually freezer) is at (-28.5 "C) so this probably is a commercial meat packing froezer. The exhaust is generally vented to the outside so as to not heat the building too much.
(a) What is the best coefficient of performance for a refrigerator that cools an environment at -28.5°C and has heat transfer to another environment at 46.5°C? 3.262 (b) How much work in joules must be done for a heat transfer of 4186 k) from the cold environment? 1283.26 (c) What is the cost (in cents) of doing this if the work costs 15.0 cents per 3.60 x 106 J (a kilowatt-hour)? 5.35 (d) How many k) of heat transfer occurs into the warm environment? 5469 26 v k) (e) Discuss what type of refrigerator might operate between these temperatures. The inside of the refrigerator (actually freezer) is at (-28.5 "C) so this probably is a commercial meat packing froezer. The exhaust is generally vented to the outside so as to not heat the building too much.
Chapter4: The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 31P: If a refrigerator discards 80 J of heat per cycle and its coefficient of performance is 6.0, what...
Related questions
Question
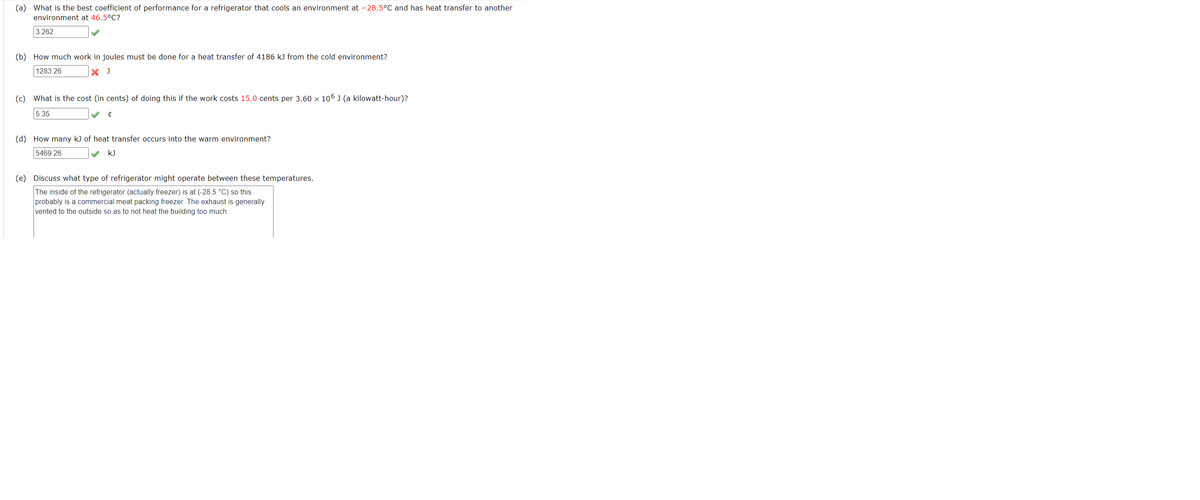
Transcribed Image Text:(a) What is the best coefficient of performance for a refrigerator that cools an environment at -28.5°C and has heat transfer to another
environment at 46.5°C?
3.262
(b) How much work in joules must be done for a heat transfer of 4186 kJ from the cold environment?
1283.26
(c) What is the cost (in cents) of doing this if the work costs 15.0 cents per 3.60 x 106 J (a kilowatt-hour)?
5.35
(d) How many k) of heat transfer occurs into the warm environment?
5469.26
kJ
(e) Discuss what type of refrigerator might operate between these temperatures.
The inside of the refrigerator (actually freezer) is at (-28.5 °C) so this
probably is a commercial meat packing freezer. The exhaust is generally
vented to the outside so as to not heat the building too much.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College