A) What is the formula of magnesium melonite dihydrate? B) What is the mass % of melon in copper (II) dimelonate? C) How many grams of melon can be obtained from 2.56 g from copper (II) dimelonate?
A) What is the formula of magnesium melonite dihydrate? B) What is the mass % of melon in copper (II) dimelonate? C) How many grams of melon can be obtained from 2.56 g from copper (II) dimelonate?
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter4: Forces Between Particles
Section: Chapter Questions
Problem 4.106E: Which of the following species will combine with a chloride ion to produce ammonium chloride? a.NH3...
Related questions
Question
A) What is the formula of magnesium melonite dihydrate?
B) What is the mass % of melon in copper (II) dimelonate?
C) How many grams of melon can be obtained from 2.56 g from copper (II) dimelonate?
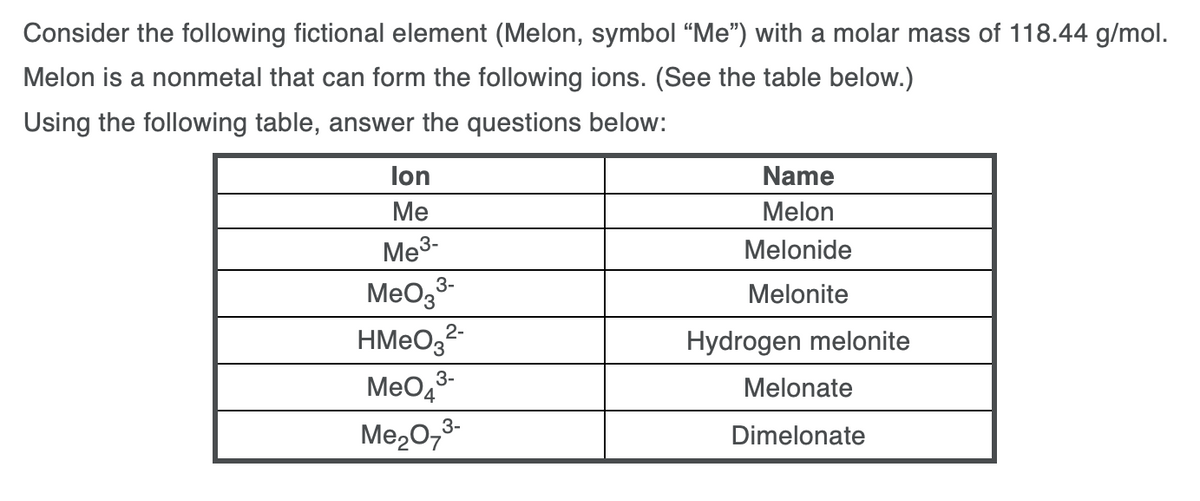
Transcribed Image Text:Consider the following fictional element (Melon, symbol “Me") with a molar mass of 118.44 g/mol.
Melon is a nonmetal that can form the following ions. (See the table below.)
Using the following table, answer the questions below:
lon
Name
Ме
Melon
Me3-
Melonide
3-
MeOg
HMEO3²-
Meo,3-
Melonite
Hydrogen melonite
Melonate
Me20,3-
Dimelonate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax