a What is the maximum mass of ammonia that can be produced from a mixture of 1.00 x 10 g N2 and 5.60 x 102 g H2? Mass = 1216.01 The strategy we will generally use to solve limiting reactant problems is to assume each reactant is limiting, and then calculate the quantity of product each reactant could produce if it were limiting. The reactant that produces the smallest quantity of product is the limiting reactant (runs out first) and therefore determines the mass of product that can be produced. Assuming N2 is limiting: 1 mol N2 28.02 g N2 17.03 g NH3 2 mol NH3 x mol N2 1.00 x 10° g N2 × mol NH3 = 1.22 x 103 g NH3 Assuming H2 is limiting: 1 mol H2 2.016 g H2 2 mol NH3 17.03 g NH3 mol NH3 5.60 x 10° g H, × 3 mol H2 = 3.15 x 103 g NH, Because N2 produces the smaller mass of product (1220 g vs. 3150 g NH3), N2 is limiting and 1220 g NH3 can be produced. As soon as 1220 g of NH3 is produced, all of the N2 has run out. Even though we have enough H2 to produce more product, there is no more N2 present as soon as 1220 g of NH3 have been produced. b What mass of which starting material would remain unreacted? g of nitrogen v would remain unreacted.
a What is the maximum mass of ammonia that can be produced from a mixture of 1.00 x 10 g N2 and 5.60 x 102 g H2? Mass = 1216.01 The strategy we will generally use to solve limiting reactant problems is to assume each reactant is limiting, and then calculate the quantity of product each reactant could produce if it were limiting. The reactant that produces the smallest quantity of product is the limiting reactant (runs out first) and therefore determines the mass of product that can be produced. Assuming N2 is limiting: 1 mol N2 28.02 g N2 17.03 g NH3 2 mol NH3 x mol N2 1.00 x 10° g N2 × mol NH3 = 1.22 x 103 g NH3 Assuming H2 is limiting: 1 mol H2 2.016 g H2 2 mol NH3 17.03 g NH3 mol NH3 5.60 x 10° g H, × 3 mol H2 = 3.15 x 103 g NH, Because N2 produces the smaller mass of product (1220 g vs. 3150 g NH3), N2 is limiting and 1220 g NH3 can be produced. As soon as 1220 g of NH3 is produced, all of the N2 has run out. Even though we have enough H2 to produce more product, there is no more N2 present as soon as 1220 g of NH3 have been produced. b What mass of which starting material would remain unreacted? g of nitrogen v would remain unreacted.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 139QRT
Related questions
Question
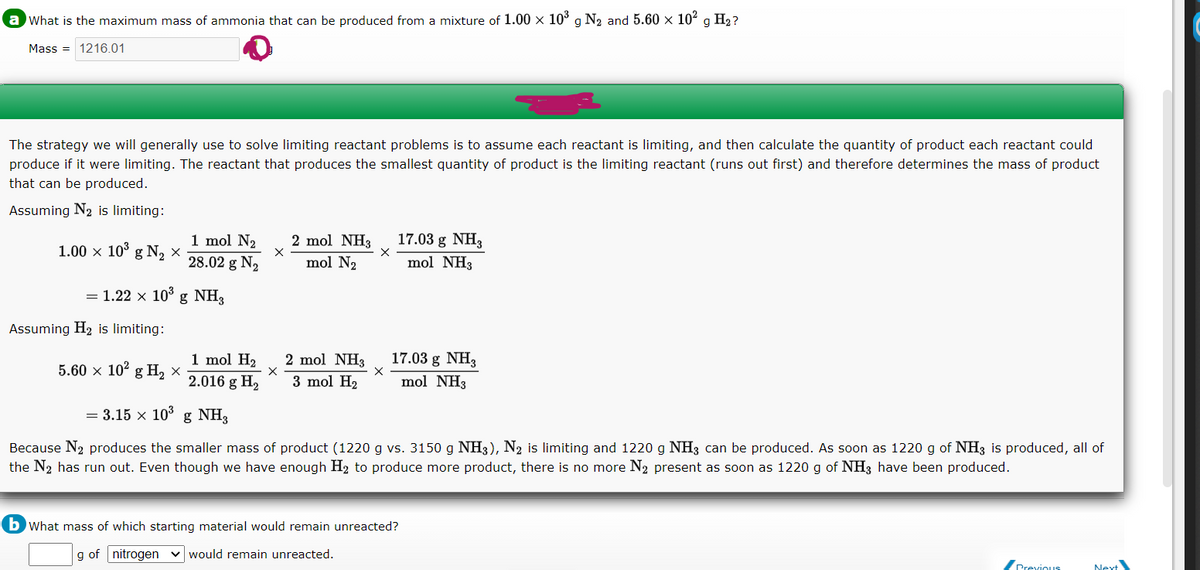
Transcribed Image Text:a What is the maximum mass of ammonia that can be produced from a mixture of 1.00 x 10° g N2 and 5.60 x 102 g H2?
Mass = 1216.01
The strategy we will generally use to solve limiting reactant problems is to assume each reactant is limiting, and then calculate the quantity of product each reactant could
produce if it were limiting. The reactant that produces the smallest quantity of product is the limiting reactant (runs out first) and therefore determines the mass of product
that can be produced.
Assuming N2 is limiting:
1 mol N2
28.02 g N2
2 mol NH3
17.03 g NH3
mol NH3
1.00 × 10° g N, ×
mol N2
= 1.22 x 103 g NH,
Assuming H2 is limiting:
17.03 g NH3
1 mol H2
2.016 g H2
2 mol NH3
5.60 x 10° g H, х
3 mol H2
mol NH3
= 3.15 x 10° g NH3
Because N2 produces the smaller mass of product (1220 g vs. 3150 g NH3), N2 is limiting and 1220 g NH3 can be produced. As soon as 1220 g of NH3 is produced, all of
the N2 has run out. Even though we have enough H2 to produce more product, there is no more N2 present as soon as 1220 g of NH3 have been produced.
b What mass of which starting material would remain unreacted?
g of nitrogen v would remain unreacted.
Previous
Next
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning