(Based on 7.6.33. from the book) The method of carbon dating makes use of the fact that all living organisms contain two isotopes of carbon, carbon-12, denoted 12C (a stable isotope), and carbon-14, denoted 14C (a radioactive isotope). The ratio of the amount of 14C to the amount of 12C is essentially constant in a living organism (approximately 1 to 1 trillion). When an organism dies, the amount of 12C present remains unchanged, but the 14C decays at a rate proportional to the amount present with a half-life of approximately 5700 years. This change in the amount of 14C relative to the amount of 12C makes it possible to estimate the time at which the organism lived. Let A, be the amount of 14C present in some living organism just (a) before it dies. Write an equation for A(t), the amount of 14C present in a dead organism t years after it dies. (b) Suppose that for some fossil found in an archaeological dig the ratio of the amount of 14C to the amount of 12C of is approximately 1 to 5 trillion. What is the approximate age of the fossil? Let Rx 10-12 be the approximate ratio of the amount of 14C to the amount of 12C measured in some fossil (so R = 0.2 in (b)). Show that amount, A(t), of 14C present in this fossil is A, · R. Hint: explain why (c) Ao original amount of 14C 10-12 A(t) current amount of 14C Rx 10-12* (d) Conclude that the age of this fossil is t = -5700 In(R)/In(2). years.
(Based on 7.6.33. from the book) The method of carbon dating makes use of the fact that all living organisms contain two isotopes of carbon, carbon-12, denoted 12C (a stable isotope), and carbon-14, denoted 14C (a radioactive isotope). The ratio of the amount of 14C to the amount of 12C is essentially constant in a living organism (approximately 1 to 1 trillion). When an organism dies, the amount of 12C present remains unchanged, but the 14C decays at a rate proportional to the amount present with a half-life of approximately 5700 years. This change in the amount of 14C relative to the amount of 12C makes it possible to estimate the time at which the organism lived. Let A, be the amount of 14C present in some living organism just (a) before it dies. Write an equation for A(t), the amount of 14C present in a dead organism t years after it dies. (b) Suppose that for some fossil found in an archaeological dig the ratio of the amount of 14C to the amount of 12C of is approximately 1 to 5 trillion. What is the approximate age of the fossil? Let Rx 10-12 be the approximate ratio of the amount of 14C to the amount of 12C measured in some fossil (so R = 0.2 in (b)). Show that amount, A(t), of 14C present in this fossil is A, · R. Hint: explain why (c) Ao original amount of 14C 10-12 A(t) current amount of 14C Rx 10-12* (d) Conclude that the age of this fossil is t = -5700 In(R)/In(2). years.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter18: Nuclear Chemistry
Section: Chapter Questions
Problem 48QRT
Related questions
Question
Question in picture, thanks. Please hand wriite the solutions so I can see clearly.
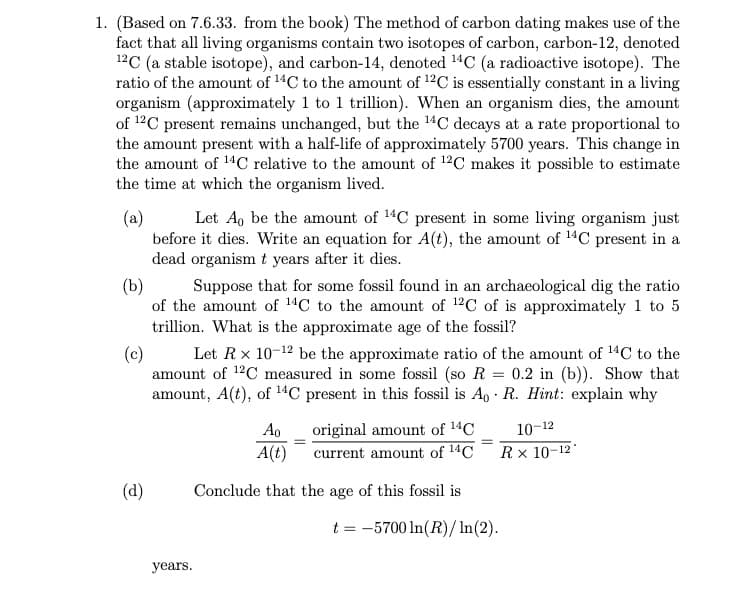
Transcribed Image Text:1. (Based on 7.6.33. from the book) The method of carbon dating makes use of the
fact that all living organisms contain two isotopes of carbon, carbon-12, denoted
12C (a stable isotope), and carbon-14, denoted 14C (a radioactive isotope). The
ratio of the amount of 14C to the amount of 12C is essentially constant in a living
organism (approximately 1 to 1 trillion). When an organism dies, the amount
of 12C present remains unchanged, but the 14C decays at a rate proportional to
the amount present with a half-life of approximately 5700 years. This change in
the amount of 14C relative to the amount of 12C makes it possible to estimate
the time at which the organism lived.
Let A, be the amount of 14C present in some living organism just
(a)
before it dies. Write an equation for A(t), the amount of 14C present in a
dead organism t years after it dies.
(b)
Suppose that for some fossil found in an archaeological dig the ratio
of the amount of 14C to the amount of 12C of is approximately 1 to 5
trillion. What is the approximate age of the fossil?
Let Rx 10-12 be the approximate ratio of the amount of 14C to the
(c)
amount of 12C measured in some fossil (so R = 0.2 in (b)). Show that
amount, A(t), of 14C present in this fossil is A, · R. Hint: explain why
original amount of 14C
10-12
A(t)
current amount of 14C
R× 10-12*
(d)
Conclude that the age of this fossil is
t = -5700 In(R)/In(2).
yеars.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 7 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning