(a) Write the half-reactions at the zinc-air electrodes and calculate the standard emf of the battery at 25°C. (b) Calculate the emf under actual operating conditions when the partial pressure of oxygen is 0.21 bar. (c) What is the energy density (measured as the energy in kilojoules that can be obtained from 1 kg of the metal) of the zinc electrode? (d) If a current of 2.1 X 10 A is to be drawn from a zinc-air battery system, what volume of air (in liters) would need to be supplied to the battery every second? Assume that the temperature is 25°C and the partial pressure of oxygen is 0.21 bar.
(a) Write the half-reactions at the zinc-air electrodes and calculate the standard emf of the battery at 25°C. (b) Calculate the emf under actual operating conditions when the partial pressure of oxygen is 0.21 bar. (c) What is the energy density (measured as the energy in kilojoules that can be obtained from 1 kg of the metal) of the zinc electrode? (d) If a current of 2.1 X 10 A is to be drawn from a zinc-air battery system, what volume of air (in liters) would need to be supplied to the battery every second? Assume that the temperature is 25°C and the partial pressure of oxygen is 0.21 bar.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 77AP
Related questions
Question
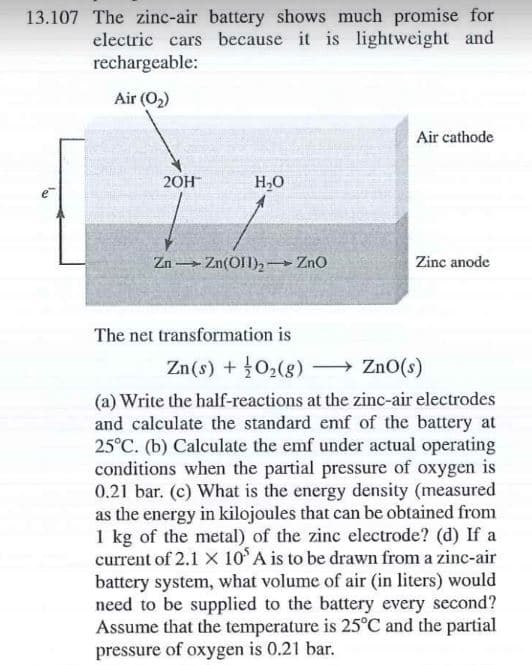
Transcribed Image Text:13.107 The zinc-air battery shows much promise for
electric cars because it is lightweight and
rechargeable:
Air (O2)
Air cathode
20H
H,0
Zn(OI1)2 ZnO
Zinc anode
Zn
The net transformation is
Zn(s) + 02(g)
» ZnO(s)
(a) Write the half-reactions at the zinc-air electrodes
and calculate the standard emf of the battery at
25°C. (b) Calculate the emf under actual operating
conditions when the partial pressure of oxygen is
0.21 bar. (c) What is the energy density (measured
as the energy in kilojoules that can be obtained from
1 kg of the metal) of the zinc electrode? (d) If a
current of 2.1 X 10 A is to be drawn from a zinc-air
battery system, what volume of air (in liters) would
need to be supplied to the battery every second?
Assume that the temperature is 25°C and the partial
pressure of oxygen is 0.21 bar.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning