a. Calculate the following: (a) volumes (in cubic meters) of the tank in each trial (Hint: formula for volume cylinder = Tr²h, where r = radius, h = height), (b) mean of the volume, (c) standard deviation of the volume, and (d) CV of the volume b. Report the average volume of this cylindrical tank together with its uncertainty considering that its average radius is 2.70 ± 0.01 m.
a. Calculate the following: (a) volumes (in cubic meters) of the tank in each trial (Hint: formula for volume cylinder = Tr²h, where r = radius, h = height), (b) mean of the volume, (c) standard deviation of the volume, and (d) CV of the volume b. Report the average volume of this cylindrical tank together with its uncertainty considering that its average radius is 2.70 ± 0.01 m.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.68QE
Related questions
Question
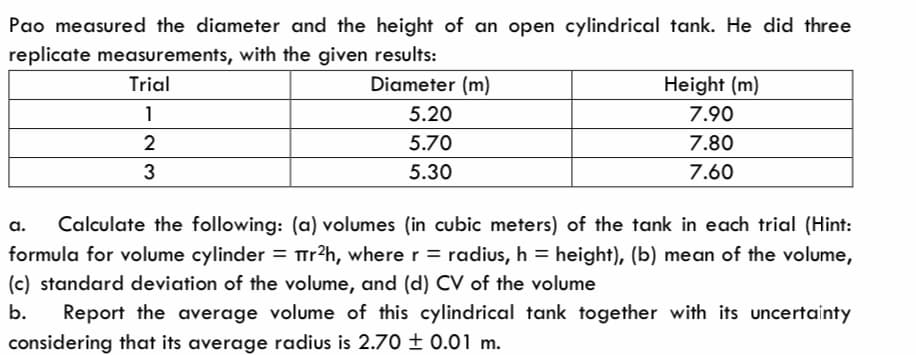
Transcribed Image Text:Pao measured the diameter and the height of an open cylindrical tank. He did three
replicate measurements, with the given results:
Trial
Diameter (m)
1
2
3
5.20
5.70
5.30
Height (m)
7.90
7.80
7.60
a. Calculate the following: (a) volumes (in cubic meters) of the tank in each trial (Hint:
formula for volume cylinder = r²h, where r = radius, h = height), (b) mean of the volume,
(c) standard deviation of the volume, and (d) CV of the volume
b. Report the average volume of this cylindrical tank together with its uncertainty
considering that its average radius is 2.70 ± 0.01 m.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning