Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.6QAP
Related questions
Question
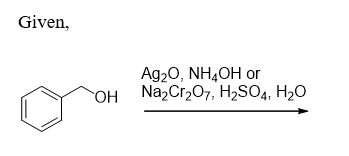
What product is formed when each compound is treated with either Ag2O, NH4OH or Na2Cr2O7, H2SO4, H2O?
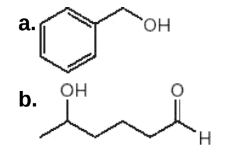
Transcribed Image Text:a.
HO,
b.
Он
H.
Expert Solution
Step 1
Since among the above reagents , Na2Cr2O7, H2SO4, H2O is a strong oxidising agent
Hence they will oxidise the terminal alcohol or aldehyde into carboxylic acid and secondary alcohol to ketones
And since Ag2O, NH4OH only oxidises aldehydes into the carboxylic acids
Hence the alcohol will remain as it is in Ag2O, NH4OH case.
Step 2
(a)
The product of the reaction has to be given.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

