A. Lewis Structure Worksheet Directions: Inside each box, draw the Lewis structure of each compound. A sample is shown: electron bond lone pair of electrons NO3 :ö: PH, H,S NH, H20 Cl, H2 SiF. NCI, SCl, он
A. Lewis Structure Worksheet Directions: Inside each box, draw the Lewis structure of each compound. A sample is shown: electron bond lone pair of electrons NO3 :ö: PH, H,S NH, H20 Cl, H2 SiF. NCI, SCl, он
Chapter8: Bonding: General Concepts
Section: Chapter Questions
Problem 7RQ: Define formal charge and explain how to calculate it. What is the purpose of the formal charge?...
Related questions
Question
100%
if possible draw it in bondpaper and please make the pic clear pls help
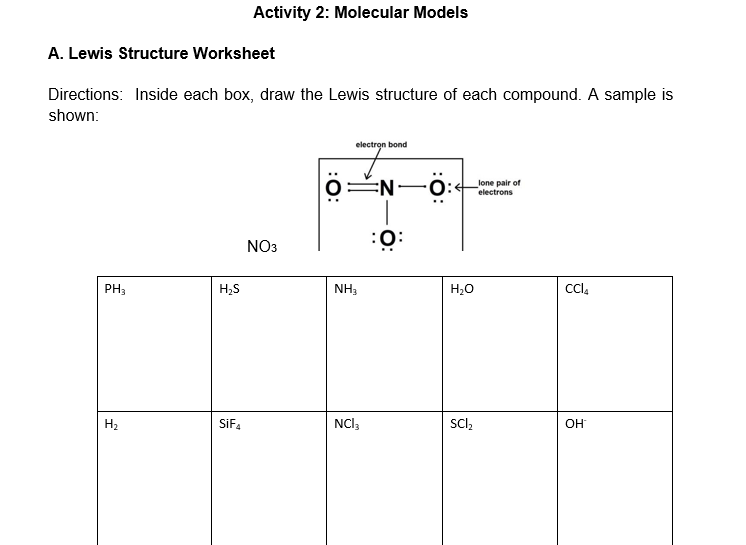
Transcribed Image Text:Activity 2: Molecular Models
A. Lewis Structure Worksheet
Directions: Inside each box, draw the Lewis structure of each compound. A sample is
shown:
electron bond
lone pair of
electrons
NO3
PH3
H;S
NH3
H20
Cla
H2
SIF4
NCI3
SCl,
OH
:0:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning