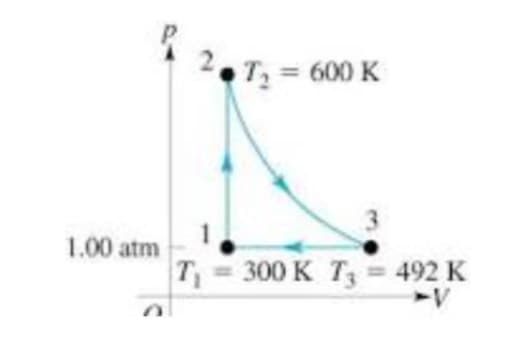
A. The following diagram represents a reversible carnot cycle of 1mol of an ideal gas (Cv = 1.5R). Calculate the work at each step, and the total work for the cycle. (Hint: one of the steps is adiabatic) B. Determine the efficiency of this cycle.
Q: The photoelectric current in a photoelectric cell can be reduced to zero by a stopping potential of…
A: We need to compute-Maximum kinetic energy of photoeectrons(KEmax)=?The data given as-Stopping…
Q: 1) Transform A = 2psin cosa +(pcos² p-psin² ) a + pcos² þa from cylindrical to Cartesian system.…
A: We will answer the question using the relations connecting coordinates and unit vectors of…
Q: What is the hydraulic radius of a stable canal carrying a discharge of 27 m³/s using Lacey's method…
A: Given: Discharge, Q = 27 m3sSilt factor, f =1.0
Q: 2. Three particles. Two protons, each with charge e, are on the y-axis and are equidistant from the…
A: Disclaimer: “Since you have asked multiple question, we will solve the first question for you. If…
Q: Date:. a car moves along a path expressed as: S = 4+² 3+² +8 2 Where s is in meters while t in…
A:
Q: 4. A toy that you mended yourself consists of a uniform bar that has two small balls glued to its…
A:
Q: A metal tank with volume 3.10 L will burst if the absolute pressure of the gas it contains exceeds…
A: We are aware that the metal tank has a 3.10 L capacity, a 100 atm absolute gas pressure, 11 mol of…
Q: A piece of metal with a mass of 1.8 kg, specific heat of 205 J/kg/°C, and initial tem- perature of…
A:
Q: 1. a) The Balmer series consists of transitions in hydrogen from higher states where n > 2 to the n…
A: Disclaimer: “Since you have asked posted a question with multiple sub-parts, we will solve the first…
Q: interpret the X-Ray Diffraction below.
A: Diffraction is well known phenomenon which gives evidence of wave nature of light. It is…
Q: : An air condenser having capacity of 50 F is charged to a potential of 200 V. If the area of each…
A: We need to find- Energy per unit volume of the field (U)=? The data given as- Capacity (C)=50…
Q: Find the maximum potential difference between two parallel conducting plates separated by 0.5 cm of…
A: given Distance d=0.5cm electricfield E=3×106v/m
Q: Q15. Find the uncertainty in the position of an electron when the mass of an electron is 9.1x10 g…
A: Given data, Mass of electron m=9.1×10-28 g=9.1×10-31 kg. Velocity of electron v=2×10-3 cm/s=2×10-5…
Q: The moment of inertia of a disc about an axis passing through its centre and perpendicular to its…
A:
Q: The flatbed railway car of mass 1200 kg travels at the constant speed around a circular path of…
A: We will first identify all the forces acting on the car and use them to answer the question. The…
Q: If you had a cylinder, sealed at both ends, with the pressure rising inside, would it blow at the…
A: We are given the pressure inside the sealed cylinder. The thing inside the cylinder is air which is…
Q: The atmosphere of Mars is mostly CO₂ (molar mass 44.0 g/mol) under a pressure of 650 Pa, which we…
A: We know, T=0°CR=8.314 J/mol·KM=44 g/molT1=-100°C The range root mean square speed of CO2 molecules…
Q: In this question we will cosider the quantum harmonic oscillator . The wavefunction of the first…
A:
Q: A baseball is thrown at an angle 30° relative to the ground with an initial velocity of 10 m/s. What…
A: Solution:-Given thatθ=30oinitial velocity (u)=10 m/stime (t)=0.5 s
Q: This question is on how we quantify the Universe. Explain how parallax is used to measure…
A: 1. The parallax angle is the angle between the Earth at one time of year, and the Earth six months…
Q: The height at which the acceleration due to gravity becomes (g /. 9) (where g = acceleration due to…
A: We need to find-Height (h)=?The data given as-At height h,gh=g9
Q: In deriving the ideal-gas equation from the kinetic-molecular model, we ignored potential energy due…
A: We must decide whether or not the exclusion of potential energy from the ideal gas equation is…
Q: A 20.0-L tank contains 4.86 × 10-4 kg of helium at 18.0°C. The molar mass of helium is 4.00g/mol.…
A:
Q: When the current in one coil changes at a rate of 5.6 A/s an emf of 6.3 × 10-3 is induced in a…
A: From question-Change in current, dIdt= 5.6 A/sand, ε=6.3×10-3 V
Q: As the drawing illustrates, two disks with masses m₁ and m₂ are moving horizontally to the right at…
A: Given,The mass of the disk 1, m1 = 1.2 kgThe mass of the disk 2, m2 = 2.2 kgThe initial velocity of…
Q: • Potential difference of the first battery ₁ = 71.0 V. • Potential difference of the second battery…
A: I_R1 = - 11.6 mA I_R2 = - 0.2 mA I_R3 = - 11.4mA
Q: 2. Mass of inner vessel with stirrer = 35 Mass of vessel with stirrer and water = 135 Mass of water…
A: Mass is fundamental property of any substance. It is unique and does not depend on external factors,…
Q: 67. In an oscillating LC circuit, the maximum charge on the capacitor is qm. Determine the charge on…
A:
Q: a 1,500 kg car tires apply 3E3 N of force against the ground. what will be the car's acceleration
A: We are given mass of car. We are also given the force applied by tires against ground. We use…
Q: A stationary circular wall clock has a face with a radius of 20 cm. Six turns of wire are wound…
A: We have given radius r = 20 cm = 20×10-2m = 0.2 m Number of turns N = 6 Current i = 1.5 A…
Q: A) Explain what the difference is between the “center of mass” and the “centroid” of an object. B)…
A: Disclaimer: “Since you have asked multiple question, we will solve the first question for you. If…
Q: A 32 lb weight stretches a spring 8 feet. The weight hangs vertically from the spring and a damping…
A: Given that: Weight, W=32 lbMass, m=Wg=3232=1 lb-s2/ftInitial equailibrium stretch, y=8 ftDamping…
Q: of liquid of density 900kg/m³ sises to a highet, of I'mm in a capillary. capillary tube of 2.4mm…
A: We need to compute-Surface tension of liquid (T)=?The data given are-Density of liquid (ρ)=900…
Q: 5. Coaxial cable. Consider the system of coaxial and concentric charges shown. A long rod carries a…
A:
Q: An electric bulb is marked 100 W. If it operates at 220 V, the resistance of the bulb will be . (a)…
A: We need to compute-Resistance of bulb (R)=?The data given as-Power of a bulb (P)=100 wattVoltage…
Q: Some stove tops are smooth ceramic for easy cleaning. If the ceramic is 0.600 cm thick and heat…
A: Given that:-Average thermal conductivity of ceramic, k=0.84 W/m°CSurface area, A=1.54×10-2…
Q: A 20.0-L tank contains 4.86 × 10-4 kg of helium at 18.0°C. The molar mass of helium is 4.00g/mol.…
A: We have to calculate the moles of helium in the tank. We are aware that the tank's volume is 20 L,…
Q: A wire of length 36 m, carrying a current of 100 A, is placed in a uniform external magnetic field.…
A: Here is the complete solution of the above problem. See below steps.
Q: As shown in the figure below, a box of mass m = 61.0 kg (initially at rest) is pushed a distance d =…
A: Given, Mass of the box, m=61kg Distance, d=83m Applied force, FA=228N The coefficient of friction,…
Q: An electric bulb is marked 100 W. If it operates at 220 V, the resistance of the bulb will be (a)…
A: We need to compute-Resistance of bulb (R)=?The data given as-Power of a bulb (P)=100 wattVoltage…
Q: The photoelectric current in a photoelectric cell can be reduced to zero by a stopping potential of…
A: We need to compute-Maximum kinetic energy of photoeectrons(KEmax)=?The data given as-Stopping…
Q: 3. Electron's trajectory. Consider an electron with an initial velocity of magnitude to directed at…
A: Disclaimer: “Since you have asked posted a question with multiple sub-parts, we will solve the first…
Q: A model rocket is constructed with a motor that can provide a total impulse of 34.0 N-s. The mass of…
A:
Q: Ex. 2: A straight conductor (rod) of length 0.4 m is rotated about one end at a constant 6280 rad/s…
A: Given,The length of the rod, l = 0.4 mThe angular frequency of rotation, ω=6280 rad/sThe uniform…
Q: Calculate binding energy of an artificial satellite orbiting at a height of 3600km surface (G=6.67X…
A: We need to compute-Binding energy (BE)=?The data given as-Mass of satellite (m)=100 kgHeight…
Q: A boy wants to throw a water balloon from a roof deck and hit the far side of the street below. He…
A: We are given the boy throwing a balloon. We are given initial velocity of ball and angle also. We…
Q: Ex/13: The mean radius of earth is 6400 km. The acceleration due to gravity at its surface is 9.8…
A: We need to compute-Massof earth (M)=?The data given as-Mean radius of earth (R)=6400 km=6400×103…
Q: Q15. Find the uncertainty in the position of an electron when the mass of an electron is 9.1×10¯ 28…
A:
Q: 220 V is supplied across 1200 windings of the autotransformer. If 1650 primary coil of the windings…
A: We have following given values-Voltage, V= 220 VoltPrimary Windings, N1= 1200Secondary Windings, N2=…
Q: A 700 pF capacitor is charged by a 50 V energy is stored in it battery. The electrostatic is (a) (c)…
A: We need to compute-Electrostatic energy stored (U)=?The data given as-Capacitance (C)=700…
A. The following diagram represents a reversible carnot cycle of 1mol of an ideal gas (Cv = 1.5R). Calculate the work at each step, and the total work for the cycle. (Hint: one of the steps is adiabatic)
B. Determine the efficiency of this cycle.
Step by step
Solved in 2 steps with 2 images
