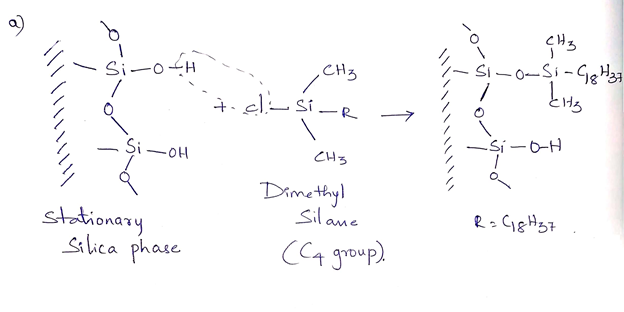
a. The structure of a standard C4 group chemically bonded to a silica surface through a silyl ether bond. b. A reasonable mechanism by which acid hydrolysis (H* and H20) might cleave the silyl ether bond, including the products of the reaction.
a. The structure of a standard C4 group chemically bonded to a silica surface through a silyl ether bond. b. A reasonable mechanism by which acid hydrolysis (H* and H20) might cleave the silyl ether bond, including the products of the reaction.
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter48: Diels-alder Reaction
Section: Chapter Questions
Problem 1Q
Related questions
Question

Transcribed Image Text:a. The structure of a standard C4 group chemically bonded to a silica surface through a
silyl ether bond.
b. A reasonable mechanism by which acid hydrolysis (H* and H20) might cleave the
silyl ether bond, including the products of the reaction.
Expert Solution
Step 1
SOLUTION:
Step 1:
The most commonly used column in silica based reverse stationary phase is octadecyl alkyl group bonded with silica (C18H37). The R groups C8, C18 and phenyl groups are used depending on the analyte and mobile phase. These alkyl groups make the column hydrophobic through covalent bonding of silica surface. These hydrophobic molecules of the mobile phase tend to adsorb to the silica stationary phase and hydrophilic molecules pass through easily and eluted first.
(a) The structure of the standard C4 group chemically bonded to silica surface through a silyl ether bond is as follows:
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole