Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 51AP: Chromium(IV) oxide is used in making magnetic recording tapes because it is paramagnetic. It can be...
Related questions
Question
Write the expected ground-expected electron configurations
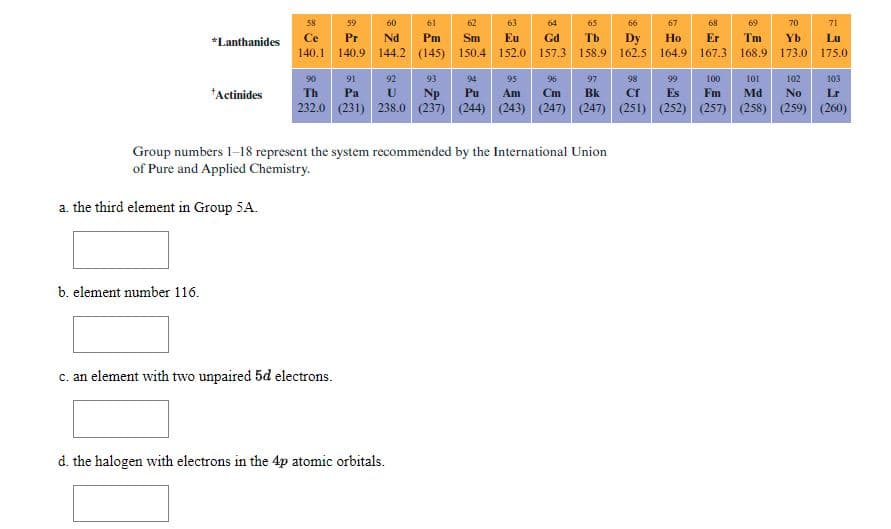
Transcribed Image Text:58
59
60
61
62
63
64
65
66
67
68
69
70
71
Ce
Pr
Dy
144.2 (145) 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.0 175.0
Nd
Pm
Sm
Eu
Gd
Tb
Но
Er
Tm
Yb
Lu
*Lanthanides
140.1
140.9
90
91
92
93
94
95
96
97
98
99
100
101
102
103
*Actinides
Th
Pa
Np
Pu
Am
Cm
Bk
Cf
Es
Fm
Md
No
Lr
232.0 (231) 238.0 (237) (244) (243) (247) (247) (251) (252) (257) (258) (259) (260)
Group numbers 1-18 represent the system recommended by the International Union
of Pure and Applied Chemistry.
a. the third element in Group 5A.
b. element number 116.
c. an element with two unpaired 5d electrons.
d. the halogen with electrons in the 4p atomic orbitals.
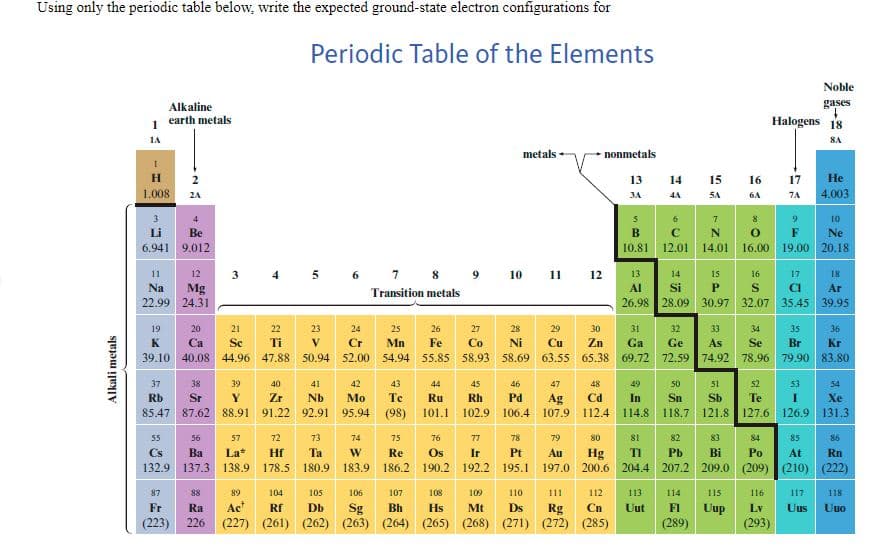
Transcribed Image Text:Using only the periodic table below, write the expected ground-state electron configurations for
Periodic Table of the Elements
Noble
Alkaline
gases
1 earth metals
Halogens 18
1A
SA
metals
nonmetals
2
13
14
15
16
17
Не
1.008
2A
ЗА
4A
5A
6A
7A
4.003
3
4
7
8
10
Li
Be
B
Ne
6.941 9.012
10.81
12.01 14.01 16.00 19.00 20.18
3 4 5 6 7 8 9
Mg
11
12
10
11
12
13
14
15
16
17
18
Na
Al
Si
S
Ar
Transition metals
22.99 24.31
26.98 28.09 30.97 32.07 35.45 39.95
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
K
Ca
Se
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.93 58.69 63.55 65.38 69.72 72.59 74.92 78.96 79.90 83.80
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
Rb
Sr
Zr
Mo
Te
Pd
Ag
107.9 112.4 114.8
Y
Nb
Ru
Rh
Cd
In
Sn
Sb
Te
Xe
85.47 87.62 88.91 91.22 92.91 95.94
(98) 101.1
102.9
106.4
118.7
121.8 127.6 126.9
131.3
5
56
57
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
Cs
Ва
La*
Hf
Ta
W
Re
Os
Ir
Pt
Au
Hg
TI
Pb
Bi
Po
At
Rn
132.9 137.3 138.9 178.5 180.9
183.9 186.2 190.2 192.2 195.1
197.0 200.6 204.4 207.2 209.0 (209) (210) (222)
87
88
89
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
Fr
Ac
Db
Sg
(263) (264) (265) (268) (271) (272) (285)
Ra
Rf
Bh
Hs
Mt
Ds
Rg
Cn
Uut
FI
Uup
Lv
Uus
Uuo
(223)
226
(227) (261) (262)
(289)
(293)
Alkali metals
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning