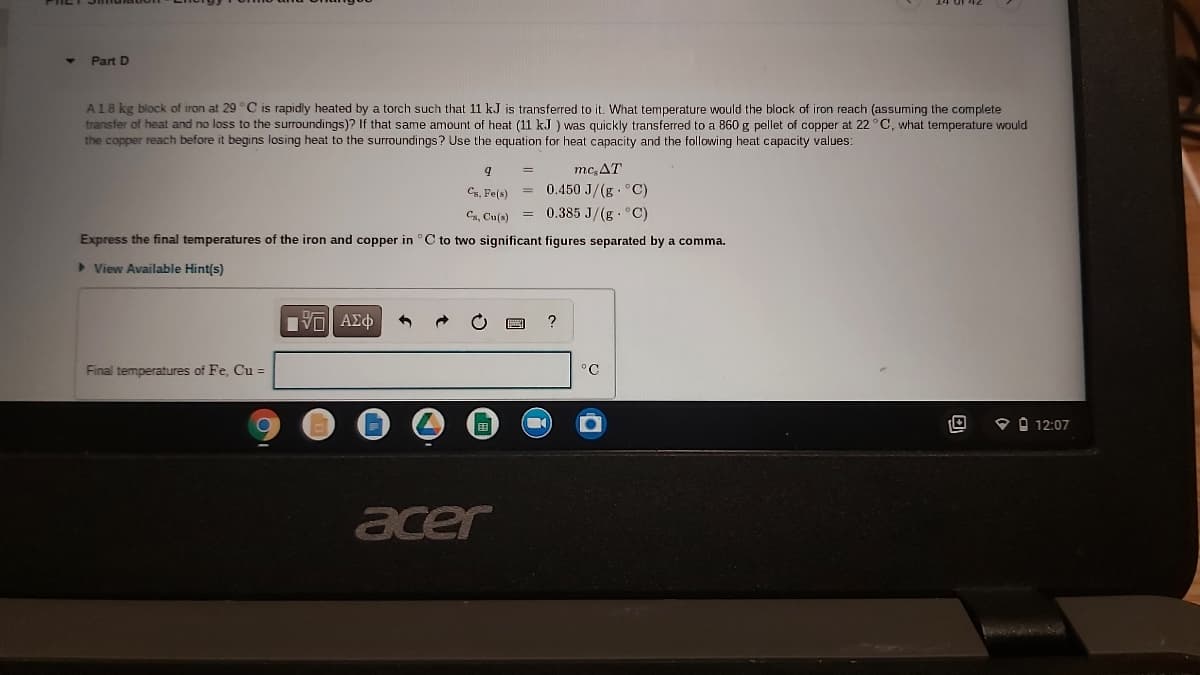
A18 kg block of iron at 29 °C is rapidly heated by a torch such that 11 kJ is transferred to it. What temperature would the block of iron reach (assuming the complete transfer of heat and no loss to the surroundings)? If that same amount of heat (11 kJ ) was quickly transferred to a 860 g pellet of copper at 22°C, what temperature would the copper reach before it begins losing heat to the surroundings? Use the equation for heat capacity and the following heat capacity values: mc, AT %3D 0.450 J/(g C) 0.385 J/(g C) Cs, Fe(s) Cs, Cu(s) Express the final temperatures of the iron and copper in C to two significant figures separated by a comma.
A18 kg block of iron at 29 °C is rapidly heated by a torch such that 11 kJ is transferred to it. What temperature would the block of iron reach (assuming the complete transfer of heat and no loss to the surroundings)? If that same amount of heat (11 kJ ) was quickly transferred to a 860 g pellet of copper at 22°C, what temperature would the copper reach before it begins losing heat to the surroundings? Use the equation for heat capacity and the following heat capacity values: mc, AT %3D 0.450 J/(g C) 0.385 J/(g C) Cs, Fe(s) Cs, Cu(s) Express the final temperatures of the iron and copper in C to two significant figures separated by a comma.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.82PAE: 9.82 The specific heat of gold is 0.13 J g-1 K-1 and that of copper is 0.39 J g-1 K-1. Suppose that...
Related questions
Question
Transcribed Image Text:Part D
A18 kg block of iron at 29 °C is rapidly heated by a torch such that 11 kJ is transferred to it. What temperature would the block of iron reach (assuming the complete
transfer of heat and no loss to the surroundings)? If that same amount of heat (11 kJ ) was quickly transferred to a 860 g pellet of copper at 22°C, what temperature would
the copper reach before it begins losing heat to the surroundings? Use the equation for heat capacity and the following heat capacity values:
mc,AT
Cs, Fe(s)
= 0.450 J/(g°C)
Cs, Cu(s) = 0.385 J/(g °C)
Express the final temperatures of the iron and copper in °C to two significant figures separated by a comma.
> View Available Hint(s)
Πνη ΑΣ Φ
Final temperatures of Fe, Cu =
°C
O A 12:07
acer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax